General Pediatrics: All Areas
General Pediatrics 5
396 - Mandated G6PD Deficiency Testing at Birth Centers in New York (NY) State - A Questionnaire Survey - How Is It Working?
Publication Number: 396.314
- PS
Pinchi Srinivasan, MD, MBA
Director, Neonatology
Icahn School of Medicine at Mount Sinai
NEW HYDE PARK, New York, United States
Presenting Author(s)
Background: Newborn Screening (NBS) for G6PD Deficiency (G6PDD) is not a standard practice in the US. In June 2022 NY State mandated testing for G6PDD. G6PDD was not added in NBS (PKU) cards. Instead, the law required newborns (NBs) be given a diagnostic test for G6PDD in NBs with “hemolytic anemia, hemolytic jaundice, early onset increasing neonatal jaundice persisting > 1st week, post discharge (DC) admissions for Jaundice and having a familial, racial, or ethnic risk of G6PDD (African, Asian, Mediterranean, or Middle Eastern ancestry)”.
Objective: Pediatric providers were surveyed electronically (SF) to learn about the experiences and challenges created by this mandate. Areas surveyed included implementation, follow up of results, use of risk criteria for testing and use of the Quantitative Assay (QA).
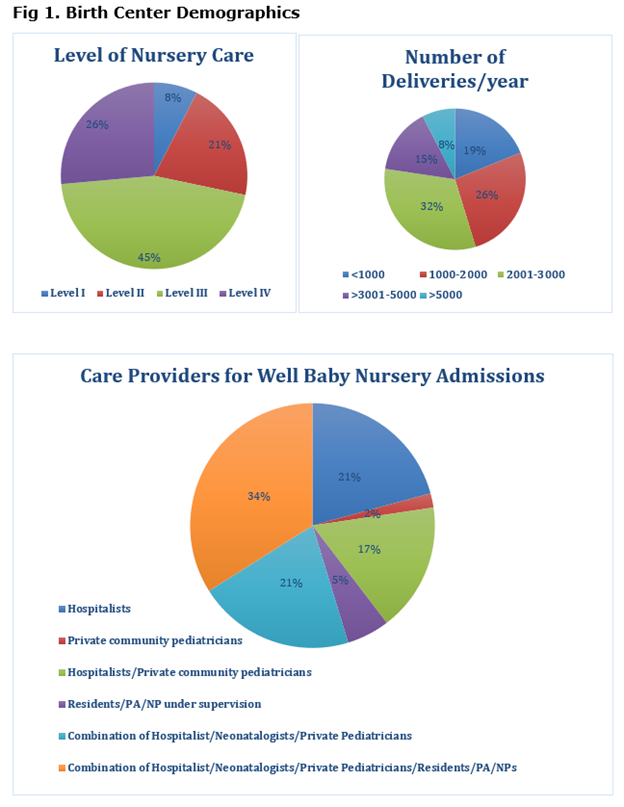
Design/Methods: Online anonymous SF on Microsoft Forms included the following topics. A. Birth Center Demographics, B. G6PD testing Mandate Implementation (plan and practices), C. G6PD QA testing and D. Logistics/challenges. SFs were sent to 123 hospitals offering (Level 1-45, Level 2-26, Level 3-34, and RPCs -17) maternity services.
Results:
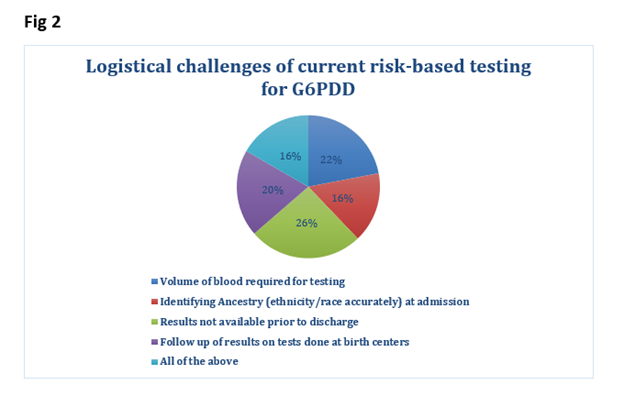
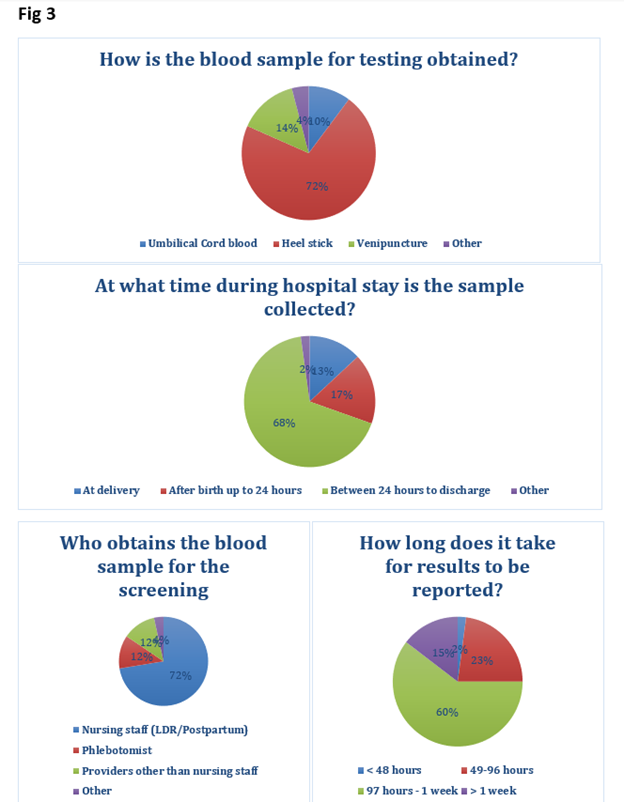
53 SF responses were completed. A. Birth Center Demographics (level of nursery, deliveries/year, and care providers for WBN) - shown in Fig1. B. Testing had been implemented in 87%. All NBs tested in 41% and risk-based criteria used in 53%. C. Quantitative assay was used in 84% and qualitative assays in 14%. D. Logistical challenges (Fig 2,3) included: nonavailability of test results before DC (71%), high volume of blood needed for testing (60%), responsibility for post DC f/u of test results (54%). Most responders (90%) preferred G6PD testing to be part of NYS NBS. 69% reported a < 1% positivity rate for testing. Under QA testing specifically logistics/challenges included a). testing sample type: heel stick (71%), venipunctures (14%), cord blood (10%), b). timing of testing: Majority (67%) between 24 hrs to DC, c). Staff collecting blood- RN (85%), d). testing site: majority sent to a reference lab (88%), e). test results available < 48 hrs in 2%, and after 4 days in 74%.
Conclusion(s): Targeted testing for G6PDD appears to have significant logistical challenges with implementation and appropriate follow up for NBs with significant variation as seen in this survey. Due to the lack of test results during the newborn stay, G6PD testing adds little to the management of hyperbilirubinemia. Addition to universal NBS through DNA screening in the state NBS programs may be a better approach despite results being available after 10-14 days with an established state NBS program.