Health Equity/Social Determinants of Health
Health Equity/Social Determinants of Health 7
676 - Racial Disparities Among Pediatric Hypertension Clinical Trials
Sunday, April 30, 2023
3:30 PM - 6:00 PM ET
Poster Number: 676
Publication Number: 676.318
Publication Number: 676.318
Chantel Johnson, Maria Fareri Children's Hospital at Westchester Medical Center, Valhalla, NY, United States; Sonia Solomon, Maria Fareri Children's Hospital at Westchester Medical Center, Valhalla, NY, United States; Mill Etienne, New York Medical College, Valhalla, NY, United States

Chantel Johnson, MD (she/her/hers)
Resident
Maria Fareri Children's Hospital at Westchester Medical Center
Valhalla, New York, United States
Presenting Author(s)
Background: Race is a social construct which impacts health outcomes. It is under-reported in clinical trials, making it difficult to identify and address health disparities. Within pediatrics, the highest rates of hypertension are seen in Hispanics followed by African Americans (1).
Objective: In this study, we analyze trials pertaining to hypertension with the primary outcome to assess the inclusion of racial data in pediatric clinical trials. Our secondary outcome is to assess their racial and gender diversity.
Design/Methods: This analysis consisted of pediatric hypertension clinical trial data from ClinicalTrials.gov using PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines. The search term “hypertension” was used to search clinical trials from 1998-2021. We then filtered studies to include completed interventional trials that included results. Microsoft Excel was used for data collection and analysis. A Chi-squared analysis (significance level: p value < 0.05) was performed to compare the percentage of White participants to that of other races as well as the percentage of male to female participants.
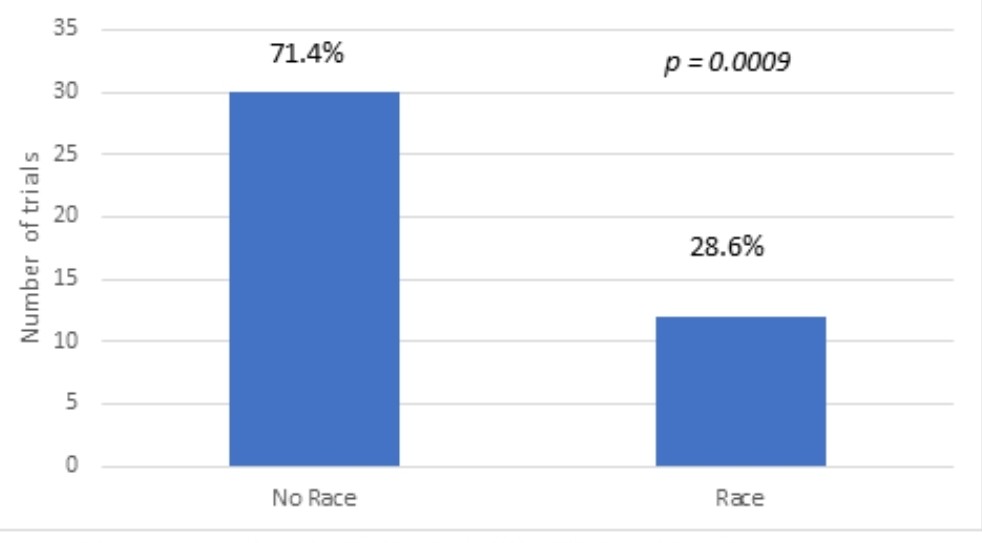
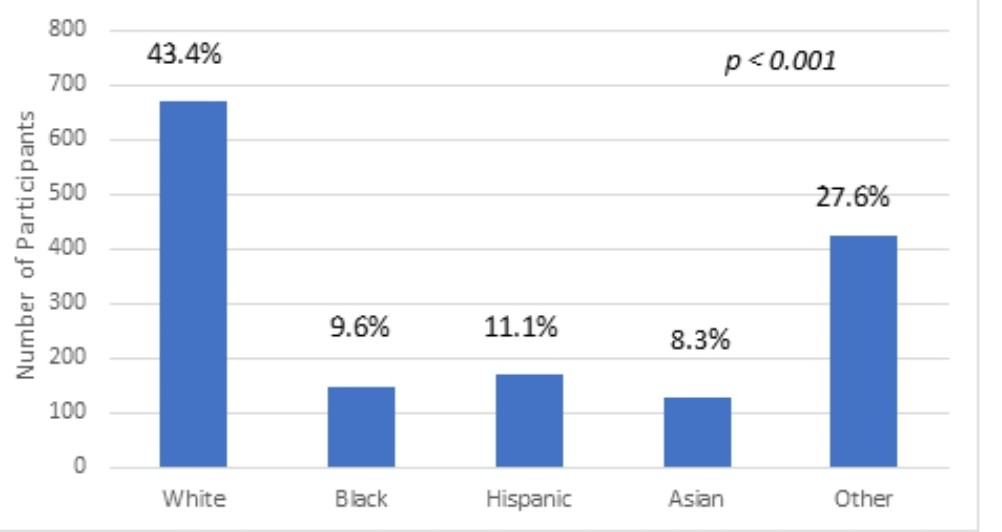
Results: The studies were from 1998-2021. 5496 trials were identified. 749 trials included children and adults. After exclusion criteria were applied, 42 trials remained. The ages of the study participants ranged between 0-22 years old. Of the 42 total pediatric hypertension trials, 12 studies (28.5 %) reported race (p=0.0009). Within the total of 4728 participants, 2195 (46.3%) were female and 2533 (53.4%) were male (p < 0.001). The 12 trials with race data had a total of 1543 participants, including 670 (43.4%) White, 148 (9.6%) Black, 171 (11.1%) Hispanic, 128 (8.3%) Asian, 426 (27.6%) “combined other” (p < 0.001). Of the 30 trials sponsored by pharmaceutical companies, six reported race, including 285 (33.7%) White, 25 (3.0%) Black, 118 (14.0%) Hispanic, 74 (8.7%) Asian, 343 (40.6 %) “combined other” (p < 0.001). In the 12 non-pharmaceutical trials, 6 mentioned race, including 383 (36.7%) Whites, 123 (11.7%) Blacks, 53 (5.1%) Hispanics, 54 (5.2%) Asians and 431 (41.3 %) “combined others” (p < 0.001).
Conclusion(s): Of the pediatric hypertension clinical trials reviewed, only 28.5% reported race. Furthermore, only 20% supported by pharmaceutical companies reported race. Women and non-White patients were significantly underrepresented in these clinical trials, which raises the concern for lack of access to these trials and generalizability of results to our Non-White pediatric patients. Further studies are needed to elucidate the underlying etiology of these disparities.
.jpg)