Neonatal Infectious Diseases/Immunology
Neonatal Infectious Diseases/Immunology 3
408 - Neonatal Gut Microbiome Composition and Gut-Derived Bloodstream Infections in a Cohort of Premature Singaporean Infants
Publication Number: 408.334
- KY
Kee Thai Yeo, MD MS FAAP FAMS (he/him/his)
Consultant
KK Women’s & Children’s Hospital
Singapore, Singapore
Presenting Author(s)
Background: Premature neonates are at high risk of bloodstream infections (BSI) caused by bacteria that reside in the gastrointestinal tract. Relatively little is known about the effect of gut microbiota alterations on the risk of BSI in premature neonates
Objective: We investigated the associations between clinical factors, gut microbiota composition and the risk of BSI in a cohort of extremely premature infants.
Design/Methods:
This prospective cohort study was conducted in a tertiary-level neonatal intensive care unit in Singapore. Clinical data and twice weekly fecal samples were collected during the first 75 days of life from infants born < 31 weeks gestational age. Fecal samples underwent shotgun metagenomic sequencing; resulting reads were aligned to Kraken 2 for taxonomic classification. We utilized permutational multivariate analysis of variance (PERMANOVA) and mixed effects linear regression to evaluate associations between clinical factors and gut microbiota composition.
Results:
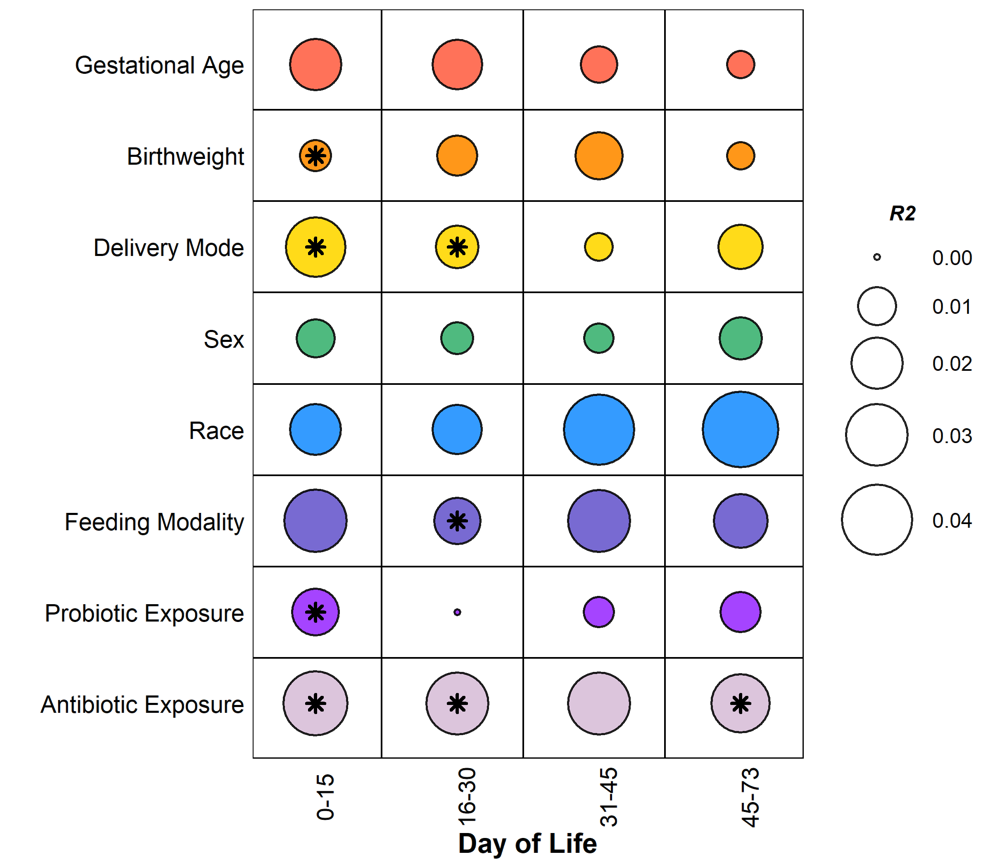
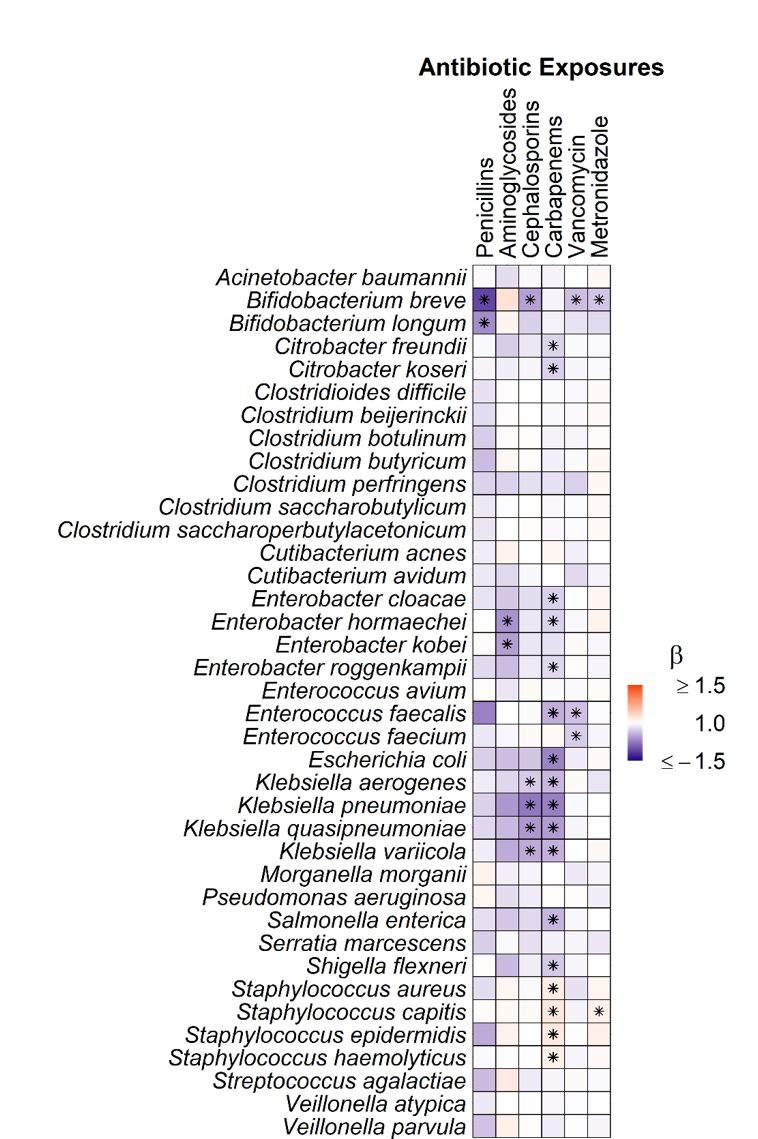
We sequenced 581 fecal samples from 75 neonates with a median (IQR) gestational age of 27 (25, 29) weeks. Metagenomic sequencing generated a median (IQR) sequencing depth of 5.9M (4.4M, 34.9M) paired-end reads per sample. Day of life (PERMANOVA; R2=0.022, p=0.001), feeding modality (R2=0.020, p=0.001), and antibiotic exposures (R2=0.018, p=0.001) accounted for the most variance in overall gut microbiota composition (Figure 1). Broad-spectrum antibiotic exposures were associated with lower relative abundances of both potential pathogens, e.g., Enterobacter, Klebsiella, and Enterococcus, and putatively beneficial bacteria, e.g., Bifidobacteria (Figure 2). Metronidazole and carbapenem exposures were associated with an increase in Staphylococcus species.
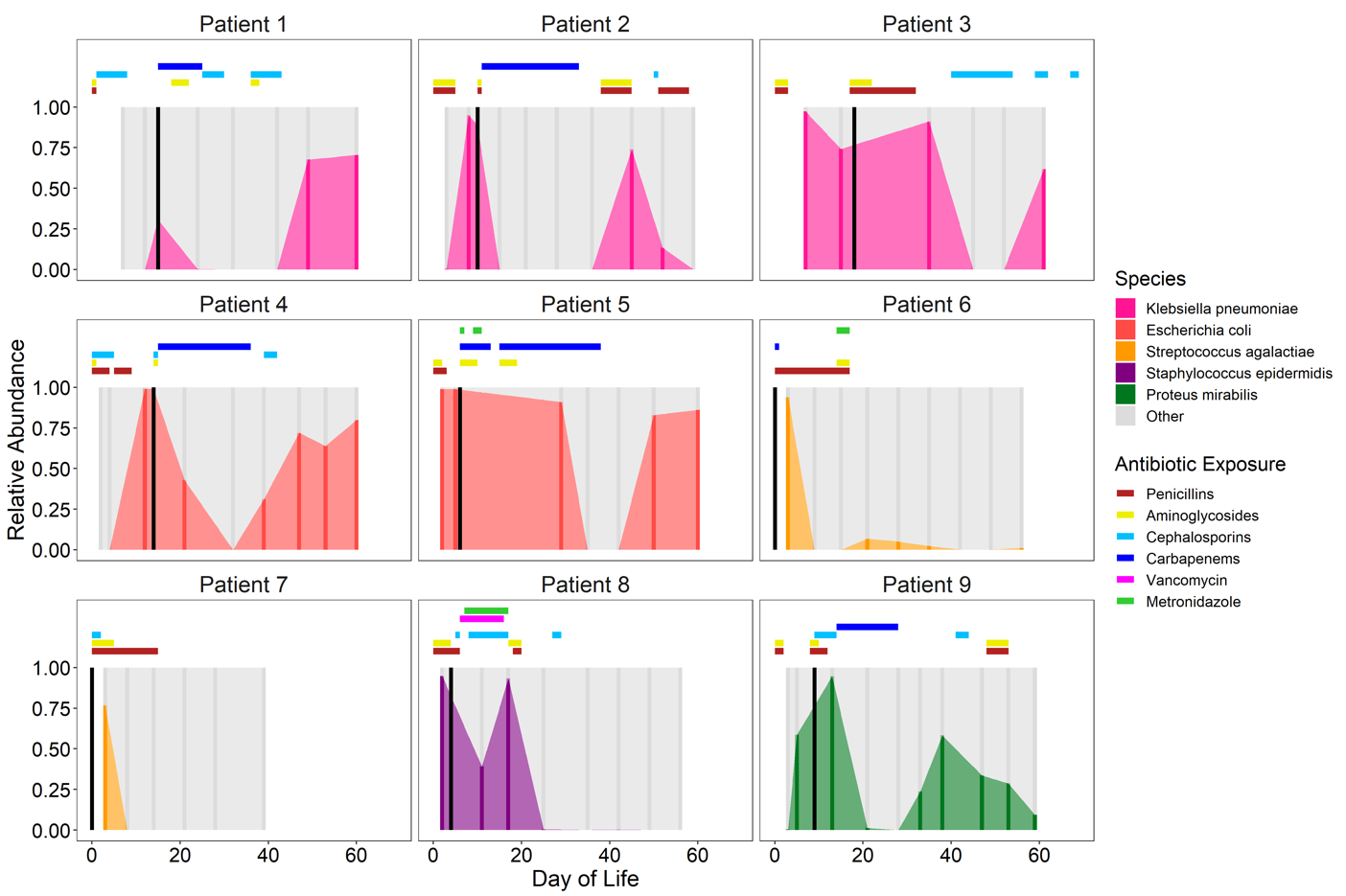
Eighteen (24%) infants developed BSI; the most frequent causative organisms were Streptococcus agalactiae (n=5), Klebsiella pneumoniae (n=3), and Staphylococcus spp. (n=4). The causative species was detected in the gut microbiota of 17 of these 18 infants. The relative abundance of the BSI species in the gut microbiota was ≥50% immediately prior to or at the time of BSI in 9 (50%) infants. Four (22%) infants had persistent high abundance of the BSI species in the gut microbiota despite definitive antibiotic therapy (Figure 3).
Conclusion(s): Several clinical factors contribute to gut microbiota composition among premature Singaporean neonates, especially postnatal age, feeding modality, and antibiotic exposures. If detected in the gut microbiota, the bacterial species that caused the BSI was often present in significant relative abundances both before and after the BSI.