Neonatal Infectious Diseases/Immunology
Neonatal Infectious Diseases/Immunology 4
426 - Screening practices for congenital cytomegalovirus: evaluation of reflexive targeted screening at a high-volume academic center
Sunday, April 30, 2023
3:30 PM - 6:00 PM ET
Poster Number: 426
Publication Number: 426.335
Publication Number: 426.335
Kristina Thompsen, Ann & Robert H. Lurie Children's Hospital of Chicago, Chicago, IL, United States; Leena B. Mithal, Ann & Robert H. Lurie Children's Hospital of Chicago, Chicago, IL, United States; Malika Shah, Ann & Robert H. Lurie Children's Hospital of Chicago, Chicago, IL, United States

Kristina Thompsen, MD (she/her/hers)
Resident Physician
Ann & Robert H. Lurie Children's Hospital of Chicago
Chicago, Illinois, United States
Presenting Author(s)
Background: Congenital cytomegalovirus (cCMV) is the most common congenitally-acquired infection and non-genetic cause of hearing loss. One screening strategy is a targeted approach based on clinical findings or failed newborn hearing screen (NBHS). There is increasing legislation in the U.S. on cCMV screening after failed NBHS, including in Illinois as of 2016 which mandates offering of cCMV testing before hospital discharge. At Prentice Women’s Hospital, a policy was implemented on June 1, 2020 to require reflexive cCMV testing after two failed NBHS.
Objective: To examine cCMV screening practices and evaluate the utility of a reflexive targeted cCMV screening policy at an urban, high-volume academic hospital.
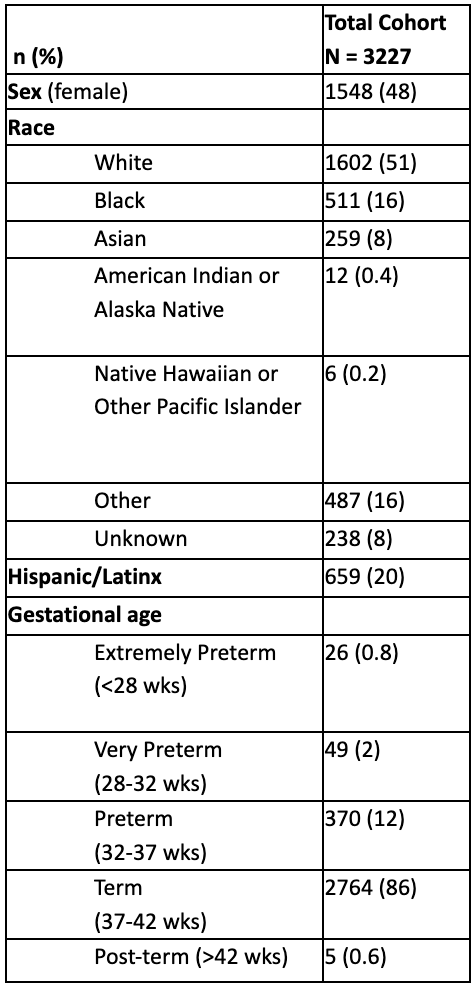
Design/Methods: Infants met inclusion criteria if they were delivered at Prentice Women’s Hospital between January 1, 2016 and August 15, 2021 and failed NBHS and/or had CMV testing during the first six months of life. Data extracted by electronic medical review (automatic through Enterprise Data Warehouse and manually) included demographics, gestational age, growth parameters at birth, NBHS results, CMV testing, laboratory/radiology studies, and antiviral treatment. Data were stratified by date of CMV testing to characterize results before and after the implementation of reflexive targeted cCMV screening.
Results: Of 49,715 infants born during the study period, 3,227 (6.5%) met inclusion criteria. From 1/1/2016 to 6/1/2020, 1,862 infants failed NBHS. Of those infants, 135 (7.3%) had a CMV test sent prior to 21 days of life and 3 infants had positive saliva CMV PCR. All 3 infants had positive confirmatory testing with urine CMV PCR, and one infant received valganciclovir treatment. After the change to a targeted screening policy (6/1/2020-8/15/2021), 416 infants failed their NBHS, 389 (93.5%) had a CMV test sent prior to hospital discharge, and 2 infants had a positive saliva CMV PCR. One of these infants had a positive confirmatory urine CMV PCR and received antiviral therapy.
Conclusion(s): A higher proportion of infants with failed NBHS were screened for cCMV after the implementation of reflexive targeted screening in June 2020 compared to prior to the policy change (93.5% versus 7.4%, respectively). As a result, a higher proportion of infants had a timely confirmed cCMV diagnosis after the 2020 protocol was implemented. Rates of cCMV were low compared to published epidemiology. At this time, confirmation of saliva screening with urine PCR remains crucial. Apart from antiviral treatment, all identified infants with cCMV will undergo prospective audiology monitoring given risk of subsequent hearing loss.