Neonatal Infectious Diseases/Immunology
Neonatal Infectious Diseases/Immunology 4
414 - Agreement Between Neonatal Cerebrospinal Fluid Glucose Measured by Standard Laboratory Method Versus Blood Gas Analyzer and Glucometer
Sunday, April 30, 2023
3:30 PM - 6:00 PM ET
Poster Number: 414
Publication Number: 414.335
Publication Number: 414.335
Sourabh Dutta, Postgraduate Institute of Medical Education and Research, Chandigarh, Chandigarh, India; Abhinay Jain, PGIMER, Chandigarh, Jind, Haryana, India; Arnab Pal, Post Graduate Institute of Medica Education and Research, Chandigarh, Chandigarh, India; Praveen Kumar, Postgraduate Institute of Medical Education and Research, Chandigarh, Chandigarh, Chandigarh, India

Sourabh Dutta, MBBS, MD, PhD (he/him/his)
Professor
Postgraduate Institute of Medical Education and Research
Chandigarh, Chandigarh, India
Presenting Author(s)
Background: Cerebrospinal fluid (CSF) glucose is used to diagnose neonatal meningitis. There is a need for a point-of-care test, since glucose levels in CSF samples decline if the analysis is delayed. Blood gas analyzers (BGA) with built-in glucose analyzers and glucometers are ubiquitous in neonatal units. There is paucity of data about the agreement between CSF glucose measured by the standard laboratory method versus measurements by BGA or glucometer.
Objective: To determine the agreement between neonatal CSF glucose measured by standard laboratory method versus BGA and glucometer
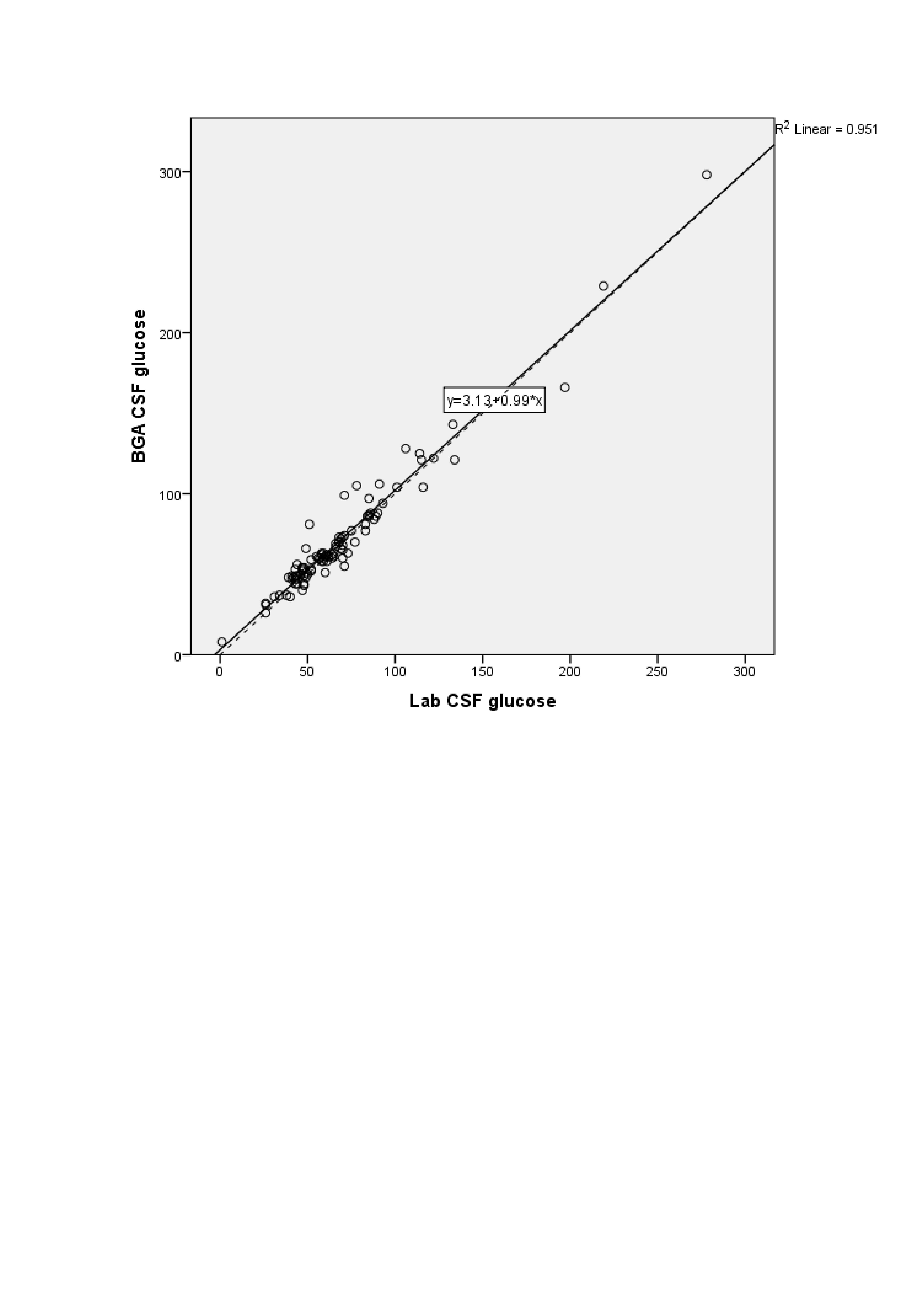
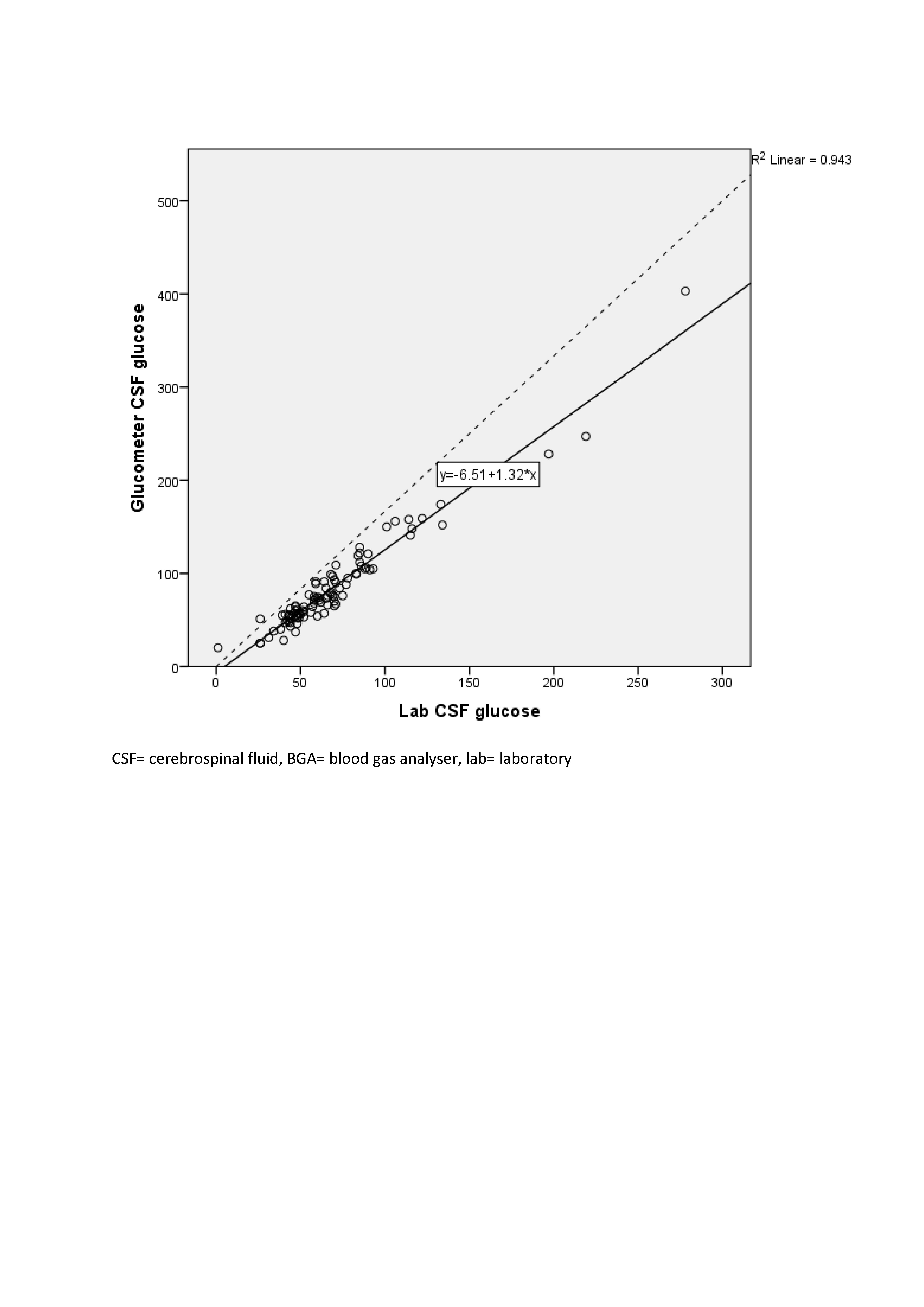
Design/Methods: In this prospective, cross-sectional study in a level III neonatal unit, neonates undergoing lumbar puncture were enrolled. CSF glucose values measured by BGA and glucometer (both index tests) were compared against values measured by the standard laboratory method performed within 45 min after LP (reference standard) from the same CSF sample. Agreement between the index tests and the reference standard was estimated by Lin’s coefficient of concordance and the Bland-Altman plot. Linear regression equations were generated to predict the standard laboratory glucose value from the BGA and glucometer values. 97 subjects were required to detect a Lin's coefficient of 0.99 with 90% power and 5% a error.
Results: 97 subjects were enrolled. Lin’s coefficient of concordance between the CSF laboratory and BGA glucose values [0.97 (95% CI: 0.96, 0.98)] was higher than that between CSF laboratory and glucometer glucose [0.87 (95% CI: 0.83, 0.90)] {P value of the difference between coefficients < 0.001}. The Bland-Altman plot of CSF glucose values by the laboratory and BGA methods showed a bias of -2.47 mg/dL (95% CI -4.20, -0.75) and limits of agreement 14.29 mg/dL to -19.23 mg/dL. The plot of laboratory versus glucometer methods showed a bias of -15.55 mg/dL (95% CI -18.86, -11.90) and limits of agreement -49.25 mg/dl to 18.49 mg/dl. A linear regression equation to predict laboratory CSF glucose from BGA values had an R2 of 0.975 [Laboratory CSF glucose= 0.35 + 0.96*(BGA CSF glucose)]. The equation to predict it from glucometer values had an R2 of 0.971 [Laboratory CSF glucose= 8.53 + 0.72*(glucometer CSF glucose)]. The median turnaround time (TAT) by the laboratory, BGA, and glucometer methods were 34 minutes (30, 37), 15 minutes (10, 18), and 3 minutes (2, 3), respectively. TAT of the laboratory method versus the BGA method (p< 0.001) and versus the glucometer method (p< 0.001) were statistically significantly different.
Conclusion(s): CSF glucose measured by blood gas analyzers may be used as a point-of-care test among neonates