Neonatal Infectious Diseases/Immunology
Neonatal Infectious Diseases/Immunology 4
424 - Pharmacoepidemiology of Ganciclovir and Valganciclovir as Treatment for Congenital Cytomegalovirus in Neonates
Publication Number: 424.335

Haejung Yoon (she/her/hers)
Medical Student
University of North Carolina at Chapel Hill School of Medicine
Cary, North Carolina, United States
Presenting Author(s)
Background: Cytomegalovirus (CMV) is the most common cause of congenital infection in the United States, affecting 4-5 per 1000 births with long term sequelae such as hearing loss, microcephaly, and chorioretinitis. There is no drug currently approved by the Food and Drug Administration for treatment of congenital CMV (cCMV) despite evidence of efficacy with ganciclovir and valganciclovir. Without a standardized treatment, clinicians continue to administer these antivirals off-label on neonates with cCMV, leading to therapy and clinical outcomes that may not be best optimized for patient needs.
Objective: To assess the pharmacoepidemiology of ganciclovir and valganciclovir for cCMV between 2010 and 2018 as well as the adverse events in neonates with cCMV who underwent treatment.
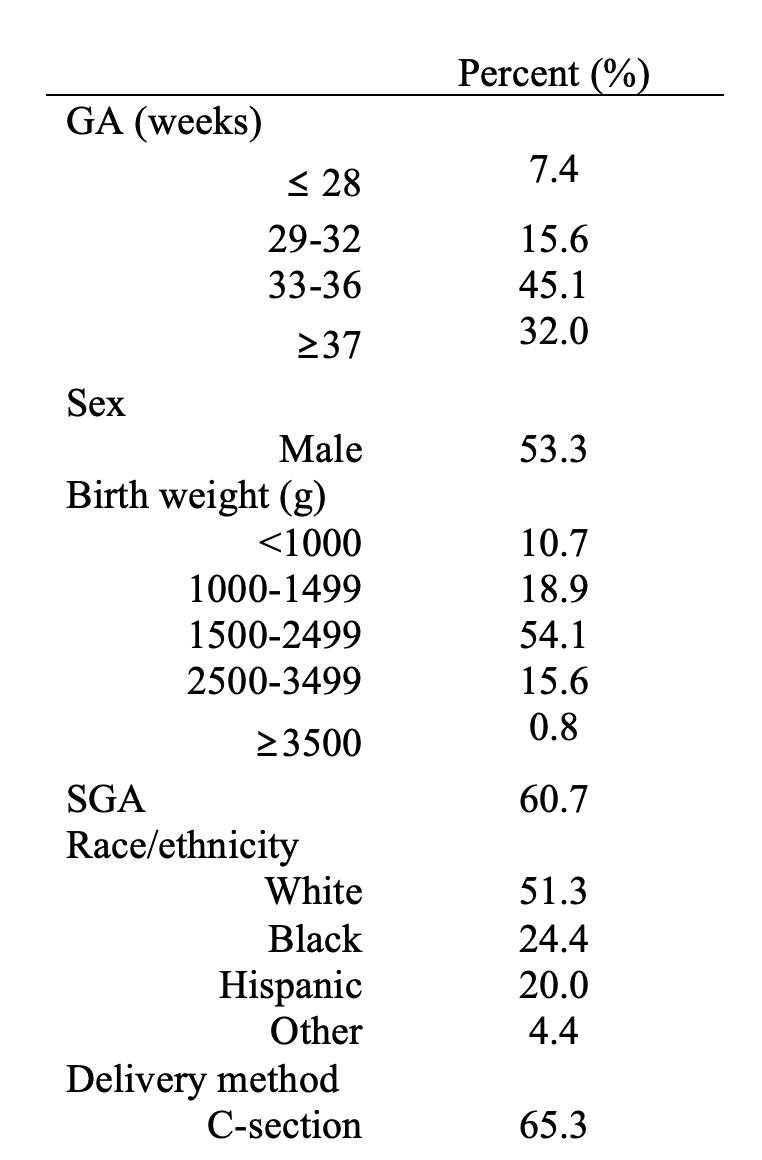
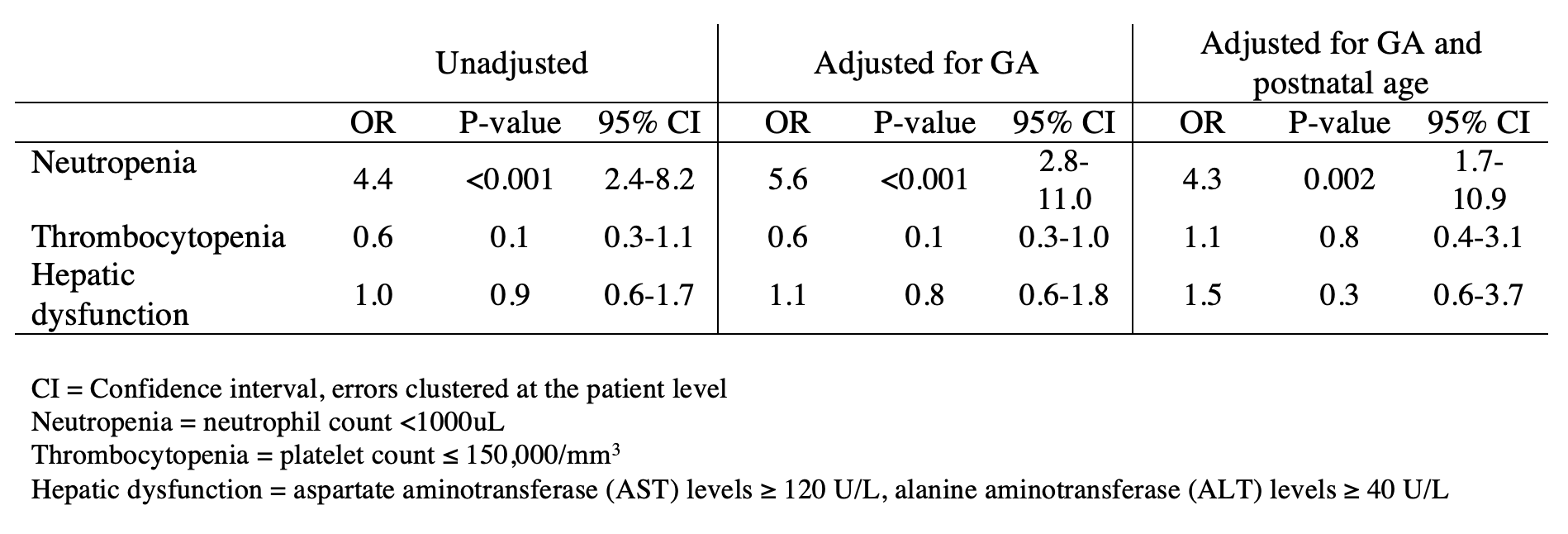
Design/Methods: We included infants discharged from Pediatrix Medical Group neonatal intensive care units (NICUs) between 2010 and 2018. We calculated the number of cCMV infants and proportion of those treated per year. We identified cCMV infants with full courses of treatment with ganciclovir and used logistic regression models adjusting for gestational age (GA) and infant age to compare adverse events (neutropenia, thrombocytopenia, and hepatic dysfunction) before and during treatment.
Results:
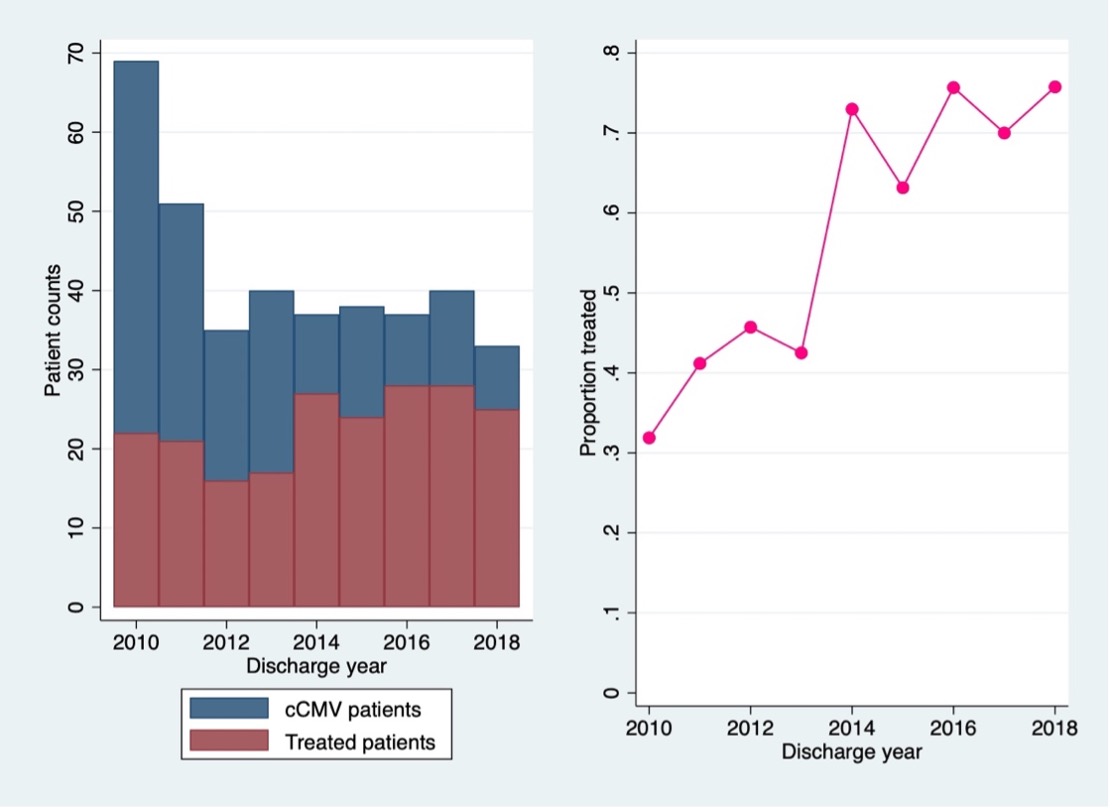
Some 380 cCMV patients from 131 sites satisfied our inclusion criteria. From 2010 to 2018, the annual number of cCMV infants fell from 69 to 33 (Figure 1, left) and the proportion of infants receiving ganciclovir rose (Figure 1, right).
Among our patients, 122 from 59 sites had sufficient treatment data for analysis of outcomes. The unadjusted odds of neutropenia during ganciclovir treatment were higher compared to before treatment (OR=4.43, p< 0.001) and when adjusted for GA (OR=5.56, p< 0.001) or for GA and infant age (OR=4.30, p=0.002). There was no statistically significant difference in the odds of thrombocytopenia or hepatic dysfunction (Table 2).
Conclusion(s): The proportion of cCMV infants treated with ganciclovir and valganciclovir has increased over time. Our data support previous studies identifying neutropenia as an adverse effect of ganciclovir. We did not find a difference in the odds of thrombocytopenia or hepatic dysfunction before and during treatment.