Health Equity/Social Determinants of Health
Health Equity/Social Determinants of Health 7
673 - Identifying Barriers to Caregiver Consent for Clinical Research Among Preterm Infants
Sunday, April 30, 2023
3:30 PM - 6:00 PM ET
Poster Number: 673
Publication Number: 673.318
Publication Number: 673.318
Yvette Chop, Nationwide Children's Hospital, Westerville, OH, United States; Carl Backes, Nationwide Children's Hospital, Columbus, OH, United States; Sara Conroy, The Ohio State University/Nationwide Children's Hospital, Columbus, OH, United States; Brian Rivera, The Abigail Wexner Research Institute at Nationwide Children's Hospital, COLUMBUS, OH, United States
- YC
Yvette Chop, MD,MPH (she/her/hers)
Fellow Physician
Nationwide Children's Hospital
Westerville, Ohio, United States
Presenting Author(s)
Background: Black birthing people in the US continue to experience the highest rates of premature delivery (2). Meanwhile, the age of fetal viability continues to decrease. Knowledge expanded by clinical trials can help optimize care for the very and extremely preterm infants. Limited literature documents racial/ethnic disparities in NICU trial enrollment(4) but paucity of data exists for these vulnerable populations. To attain appropriate representation of minoritized groups in research trials, culturally sensitive insight into enrollment barriers is urgently needed.
Objective: Identify barriers caregivers of NICU infants face when deciding if to provide consent for participation in clinical studies.
Design/Methods: The study was conducted from July 20th,2022 to December 20th, 2022 at two US NICUs. Inclusion criteria:1) being approached about research while in the NICU and 2)infant < 32 weeks gestational age when approached. Exclusion criteria:1) Caregiver < 18 years old or 2)never approached to participate in a study. For the study, a 76-question survey instrument was developed based on a previously validated scale. Caregivers of very and extremely premature infants eligible for participation in 12 trials were candidates. A subset of survey questions (13) designed to assess caregiver decision-making were analyzed.
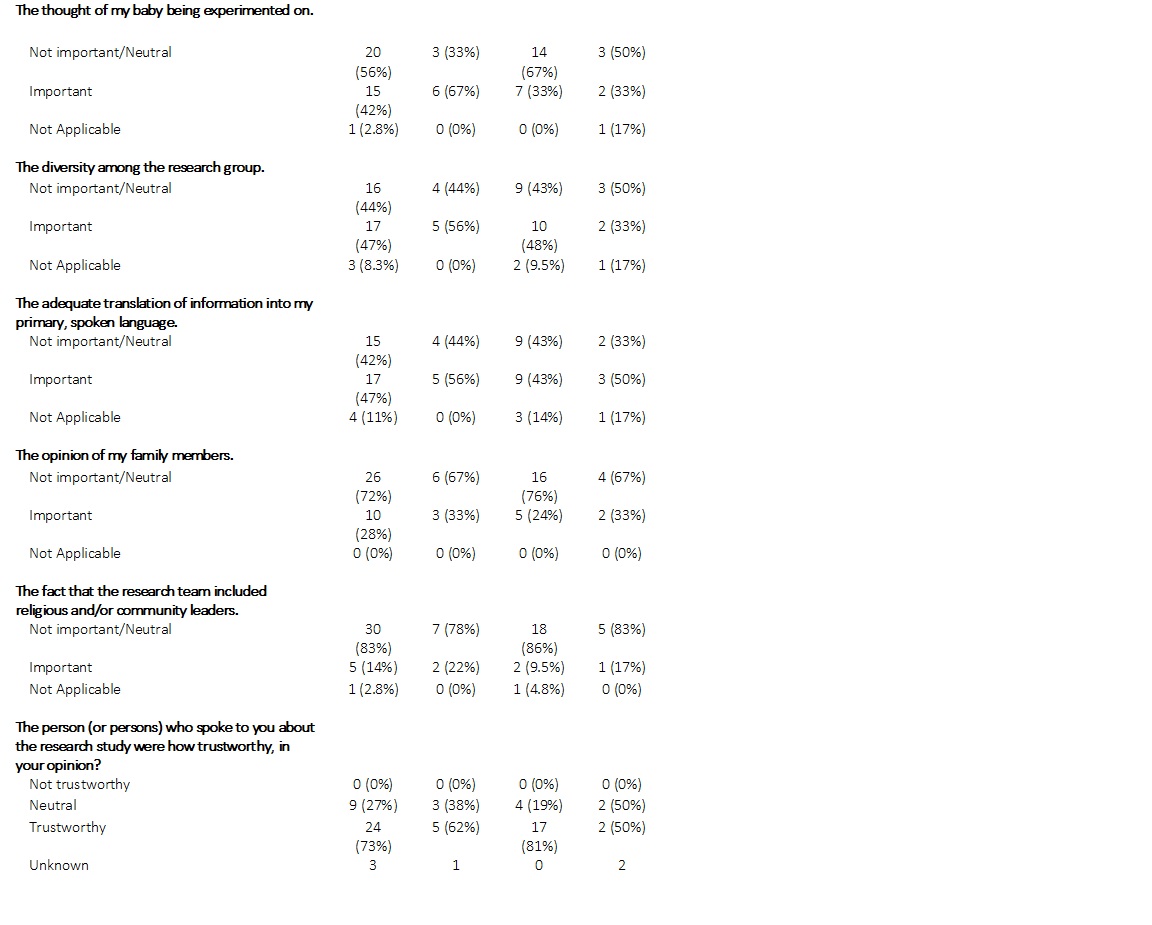
Results: Analysis was of 36 completed surveys (114 screened). Self-reported race and ethnicity data: 26% Black, 55% as White, and 2.5% Hispanic (Table 1). Furthermore, 47% had an income of $50,000 or less. We observed the following with greater than 10% difference in responses by race (Table 3): 22% of Blacks vs 4.8% of Whites felt that the research would not benefit minorities, 33% of Blacks vs 9.5% of Whites felt researchers would be protected against harm that could happen to their infant if enrolled. 67% of Blacks vs 33% of Whites viewed the thought of their baby being experimented on an important factor, while 22% of Blacks vs 9.5% of Whites viewed a research team including religious and community leaders important. 47% of respondents reported that it was important to have the information translated into their primary language (56% Black vs 43% Whites).Caregivers reported high levels of trust in those who approached them about the research(73%).
Conclusion(s): Results of this pilot study provide insight into factors associated with caregiver decision-making and research participation. This information can inform future methods to improve enrollment in neonatal clinical trials among underrepresented minoritized groups. This may assist in curtailing disparities in NICU outcomes.
.jpg)
.jpg)
