Neonatal Infectious Diseases/Immunology
Neonatal Infectious Diseases/Immunology 4
425 - Rotavirus vaccine administration to preterm infants during hospitalization: Evaluation of health care benefits and cost effectiveness analysis.
Publication Number: 425.335
Peter J. Porcelli, Jr., MD MS
Professor of Pediatrics
Wake Forest Baptist Health - Brenner Children's Hospital Atrium Baptist Health -- BCH.
Wake Forest University School of Medicine
Winston-Salem, North Carolina, United States
Presenting Author(s)
Background:
Rotavirus (RV) is the most common cause of severe intestinal viral infection in infants. Before vaccine availability, RV infection in the US accounted for 256k ER visits and $337 M (2022 USD) annual healthcare costs. RV is still common with 2.7M annual cases in the US.
Rotavirus vaccine is unique because the series must be started by 105 days of age; yet it is common for preterm infants to be hospitalized >105 days after which there is no catch-up schedule, and the infant never receives RV vaccination.
Live vaccines are shed in stool, and early recommendations (AAP 2009) discouraged inpatient RV vaccine. As a result, few neonatal care centers routinely provide live oral RV vaccine during hospitalization. Recent reports indicate low risk providing RV vaccination to hospitalized infants. In April, 2022, our nursery initiated oral RV vaccination between 60-105 days of age for NICU inpatients < 28 wk gestational age.
The CDC report of 30k ELBW infants represents 0.81% of 3.66 million US births in 2021; this value was used as a proxy for our population. Also, preterm infants utilize up to 2.2 times more healthcare resources after DC compared to term infants [Tomashek, SemPeri].
Objective:
Determine efficacy of inpatient RV vaccination with potential health benefits and cost savings of a national program.
Design/Methods:
Practice guidelines were modified to identify infants < 28 wk gestation as high risk to exceed a 105 day hospital stay and to provide oral RotaTeq vaccine between 60- 105 days of age. Infants were monitored for gastrointestinal and infectious complications. Retrospective data were collected from 4/4-12/15/2022 using internal data sources. All costs were adjusted to US$ using CPI-MED.
Results:
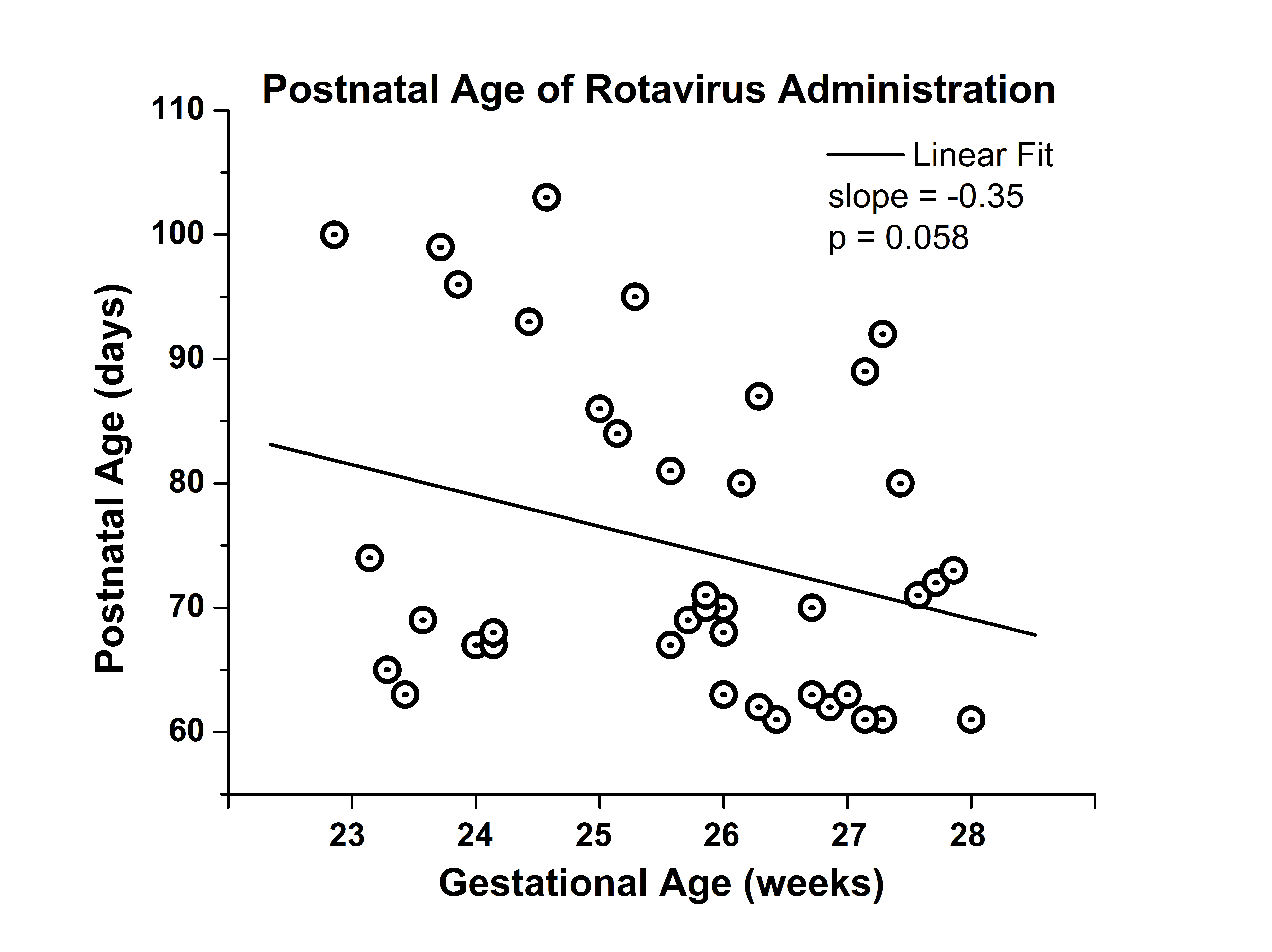
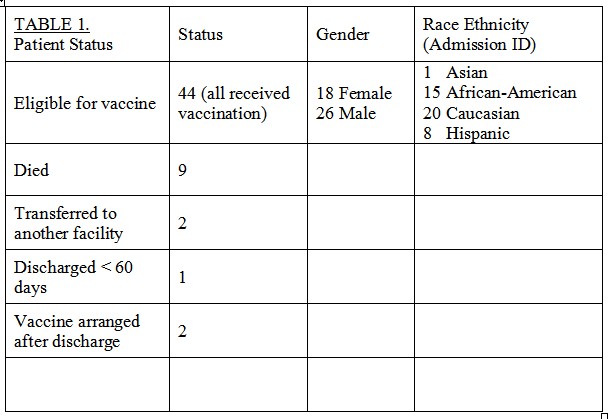
58 patients met inpatient RV vaccination guidelines and 44 received the vaccine, Table 1. Reasons for exclusion included death(9), transfer(2), early DC(1) or vaccine scheduled after DC(2). Vaccines were administered from 61-103 days of age. Age at vaccination was more variable with lower gestational age, p=0.058, Fig 1. No gastrointestinal nor cross infection issues were reported.
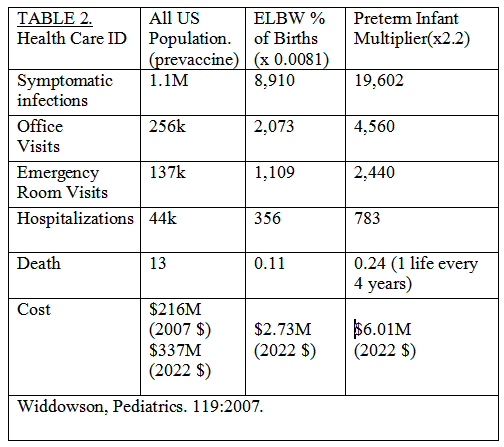
Providing RV vaccination to all 30k US ELBW infants as inpatients could prevent almost 20k infections, Table 2. Using an estimated average cost for RV vaccination of $92 shows the cost to provide the vaccine to all ELBW infants of $2.76M is less than the $6M cost savings.
Conclusion(s):
Administering RV vaccine to inpatient preterm infants is safe and could prevent post DC infections. The finding that healthcare cost savings exceeded RV vaccination costs supports further evaluation to extend RV vaccination to NICU inpatients.