Allergy, Immunology and Rheumatology
Allergy, Immunology, and Rheumatology
383 - Serum cytokine and chemokine reference values for human milk-fed infants at 4 and 6 months of life
Sunday, April 30, 2023
3:30 PM - 6:00 PM ET
Poster Number: 383
Publication Number: 383.301
Publication Number: 383.301
Kinga K. Smolen, Harvard Medical School, Boston, MA, United States; Diana Orenstein, ByHeart, Inc., New York, NY, United States; Catherine J. Field, University of Alberta Faculty of Agricultural, Food and Nutritional ScienceMedicine and Dentistry, Edmonton, AB, Canada; Devon Kuehn, ByHeart, Inc, New York, NY, United States

Devon Kuehn, MD (she/her/hers)
Chief Medical Officer
ByHeart, Inc
New York, New York, United States
Presenting Author(s)
Background: The early weeks of infancy represent a critical period in the developing immune system with lasting health implications. Alteration to the immune trajectory can increase susceptibility to infection, attenuate vaccine response, and impact the pathway of disease development. Cytokines and chemokines are signaling molecules with roles in innate and adaptive immune responses; factors such as premature birth, diet, sex, and age may modulate expression. There is a lack of reference data on early ontogeny of serum cytokine and chemokine concentrations in healthy infants due to limitations in study design.
Objective: The objective of this study was to investigate the ontogeny of cytokine and chemokine concentrations in healthy term 4- and 6-month-old human milk-fed infants.
Design/Methods: This secondary analysis was part of a trial designed to evaluate the growth and safety of a novel infant formula. Healthy term infants who were exclusively human milk-fed were enrolled as a reference group. Cytokine and chemokine data were generated using the Olink proximity extension assay in serum from 4- and 6-month-old infants. The Olink Target 48 Cytokine panel was used to detect a range of biomarkers. Unsupervised clustering was employed to summarize variability in the log2-transformed concentration. All cytokines and chemokines were centered and scaled to unit variance.
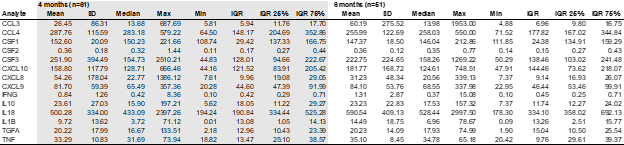
Results: Analysis included 113 samples (61 at 4 months, 51 at 6 months). Most participants were born by vaginal delivery (80.5%), primarily at 39 weeks gestation (51.8%) with 46% male. Table 1 is provided as a reference. In this interim analysis, there were no significant differences in concentrations between the two time points except IL-18 (higher at 6 months compared to 4 months; p=0.042). Trends toward differences were observed in individual cytokines and chemokines associated with clinical factors, including mode of delivery at 4 and 6 months, sex, maternal antibiotic use during delivery, and maternal BMI at 6 months. No significant differences were associated with weight, length, or head circumference.
Conclusion(s): There were no major differences in cytokine and chemokines concentrations between 4 and 6 months of life, suggesting a robust immune phase. Mode of delivery, sex, maternal antibiotic use during delivery, and maternal BMI impacts cytokine and chemokine expression. The availability of these data will support future research by allowing for the comparison of cytokine and chemokine concentrations in a cohort of healthy human milk-fed infants to concentrations in other populations, increasing understanding of early life immune development.