Neonatal Neurology: Pre-Clinical Research
Neonatal Neurology 8: Preclinical 2
20 - Blood Glucose and Ketone Levels Stratify Severity of Injury After Experimental Neonatal Hypoxia-Ischemia in Mice
Monday, May 1, 2023
9:30 AM - 11:30 AM ET
Poster Number: 20
Publication Number: 20.434
Publication Number: 20.434
Sarah Ann W. Duck, Johns Hopkins University School of Medicine, Baltimore, MD, United States; Michelle M. Nazareth, Johns Hopkins University, Baltimore, MD, United States; Salma Nassar, Johns Hopkins University, Brookfield, CT, United States; Michael Nugent, Johns Hopkins University School of Medicine, Baltimore, MD, United States; Mark St. Pierre, Johns Hopkins University School of Medicine, Baltimore, MD, United States; Susan J. Vannucci, Weill Cornell Medicine, New York, NY, United States; Raul Chavez-Valdez, Johns Hopkins University School of Medicine, CATONSVILLE, MD, United States

Sarah Ann W. Duck (she/her/hers)
Research Technician
Johns Hopkins University School of Medicine
Wilmetter, Illinois, United States
Presenting Author(s)
Background: The Vannucci procedure is widely used to model cerebral hypoxic-ischemic (HI) injury in neonatal rodents. However, injury is variable making the study of potential neuroprotective treatments challenging. Post-insult in-vivo T2W MRI may guide stratification of pups to delayed treatments, but not to immediate interventions. Identifying minimally invasive biomarkers linked to brain injury could improve stratification of pups to potential treatments.
Objective: Infants exhibiting hypoglycemia post-HI insult are more likely to have greater brain injury and disabilities (Parmentier et al., 2022). We hypothesized that blood glucose levels after HI will correlate with severity of brain injury in this model. We also measured blood ketone body (KB) levels since they are a significant cerebral fuel in neonatal rodents.
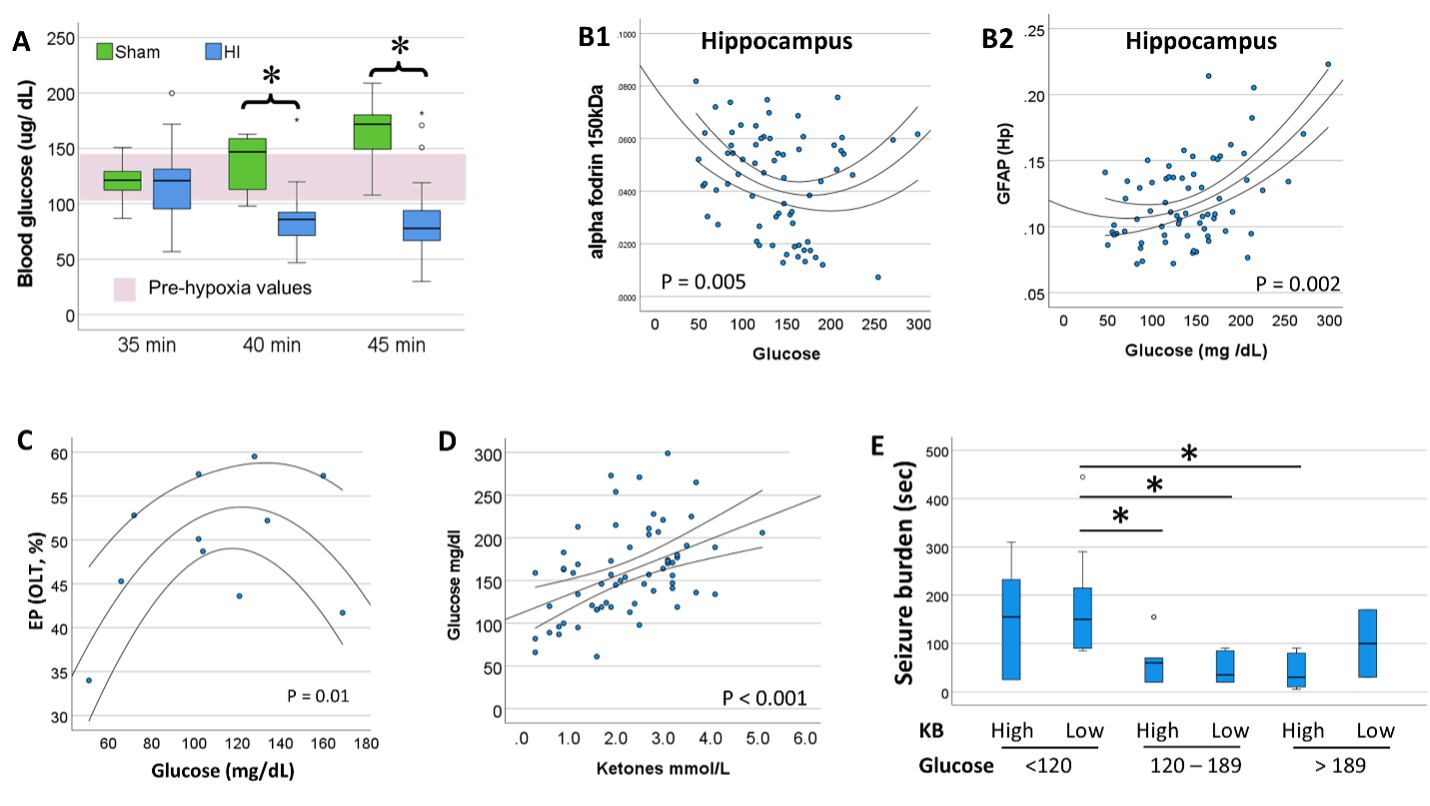
Design/Methods: C57BL6 mice of both sexes were subjected to cerebral HI at P10 with right carotid artery ligation plus hypoxia (FiO2 0.08) for 35, 40, 45 min. Clinical seizures recorded during hypoxia; blood glucose and KB levels measured immediately after hypoxia. GFAP (astrocytic activation) and α-fodrin breakdown products [BPDs] (cytoskeletal neural damage) were measured in hippocampus, thalamus, and cortex at P11 to assess injury severity. Open field (OF), Y-maze (YM), and Object-location task (OLT) were tested at P40.
Results: Glucose levels ranged between 104 to 149 mg/dL at P10 in naive mice. Longer hypoxic exposures resulted in lower blood glucose. A quadratic regression model (U-shape) better represented the relationship between glucose and GFAP and 150kDa α-fodrin levels; extreme glucose levels (high or low) were associated with greater hippocampal, thalamic, and cortical injury. Similar quadratic relationships were found between glucose and distance and time in the center of the arena (OF) and time spend exploring objects in novel location (OLT). Although, glucose and KB levels were directly correlated; ketones levels only related to GFAP and α-fodrin BDPs in combination with glucose levels. Either high ketones (≥ 2.2 mmol/L, median) w/low glucose (< 120 mg/dl, 25%til) or low ketones (< 2.2 mmol/L) w/high glucose ( > 189 mg/dl, 75%til) were associated with worse hippocampal injury and higher seizure burden. No sexual dimorphism was detected in any of these relationships.
Conclusion(s): Post-hypoxia blood glucose and KB levels are a minimally invasive screening tool to identify animals with significant HI brain injury in this mouse model. This tool improves our ability to stratify pups to experimental treatments to assess effectiveness.