Neonatal Clinical Trials
Neonatal Clinical Trials 2
277 - The TELENEO Pilot Study: Evaluating the Feasibility of a North American Multicenter Clinical Trial of Teleneonatology in Community Hospitals
Monday, May 1, 2023
9:30 AM - 11:30 AM ET
Poster Number: 277
Publication Number: 277.428
Publication Number: 277.428
Jennifer Fang, Mayo Clinic, Rochester, MN, United States; Rachel Umoren, University of Washington School of Medicine, Seattle, WA, United States; Hilary E A. Whyte, SickKids Hospital, Toronto, ON, Canada; Jamie Limjoco, American Family Children's Hospital, MADISON, WI, United States; Abhishek Makkar, Oklahoma Childrens Hospital at OU Health, Oklahoma City, OK, United States; Lauren White, Oklahoma Childrens Hospital at OU Health, Mustang, OK, United States; Marko Culjat, The Hospital for Sick Children, Toronto, ON, Canada; Sangeet Kathuria, William osler health care, Brampton, ON, Canada; Malinda Webb, Oklahoma Childrens Hospital at OU Health, Stillwater, OK, United States; Todd Schad, University of Wisconsin School of Medicine and Public Health, Sauk City, WI, United States; sue Ann. shafranski, Sauk Prairie Healthcare, Prairie du Sac, WI, United States; Supriya Behl, Mayo Clinic Children's Center, Rochester, MN, United States; Rosanna Yankanah, The Hospital for Sick Children, Toronto, ON, Canada; Jeph Herrin, Flying Buttress Associates, Charlottesville, VA, United States; Bart M. Demaerschalk, Mayo Clinic College of Medicine and Science, Scottsdale, AZ, United States
- JF
Jennifer Fang, MD, MS (she/her/hers)
Associate Professor of Pediatrics
Mayo Clinic
Rochester, Minnesota, United States
Presenting Author(s)
Background: At-risk neonates born in community hospitals without a neonatal intensive care unit (NICU) are at increased risk of mortality and serious morbidity. Synchronous audio-video telemedicine consultation with a neonatologist (termed “teleneonatology”) may improve the resuscitative care and clinical outcomes for these neonates. Our research consortium is preparing for a prospective multicenter trial evaluating the impact of teleneonatology on the early health outcomes of at-risk neonates born in community hospitals.
Objective: The objective of the TELENEO pilot study was to demonstrate feasibility of the trial protocol as measured by a composite feasibility score.
Design/Methods: Four hospitals with regional neonatal intensive care units (‘hubs’) and four community hospitals with newborn nurseries (‘spokes’) participated in the pilot – forming four hub-spoke dyads. Two hub-spoke dyads implemented teleneonatology. Neonates were enrolled from January 22, 2022, to June 30, 2022, using trial eligibility criteria. The primary outcome was a composite feasibility score that included one point for each of the following: site retention, on-time screening log completion, no eligibility errors made, on-time data submission, and sponsor site-dyad meeting attendance (calculated per hub-spoke dyad per month; score range 0-5).
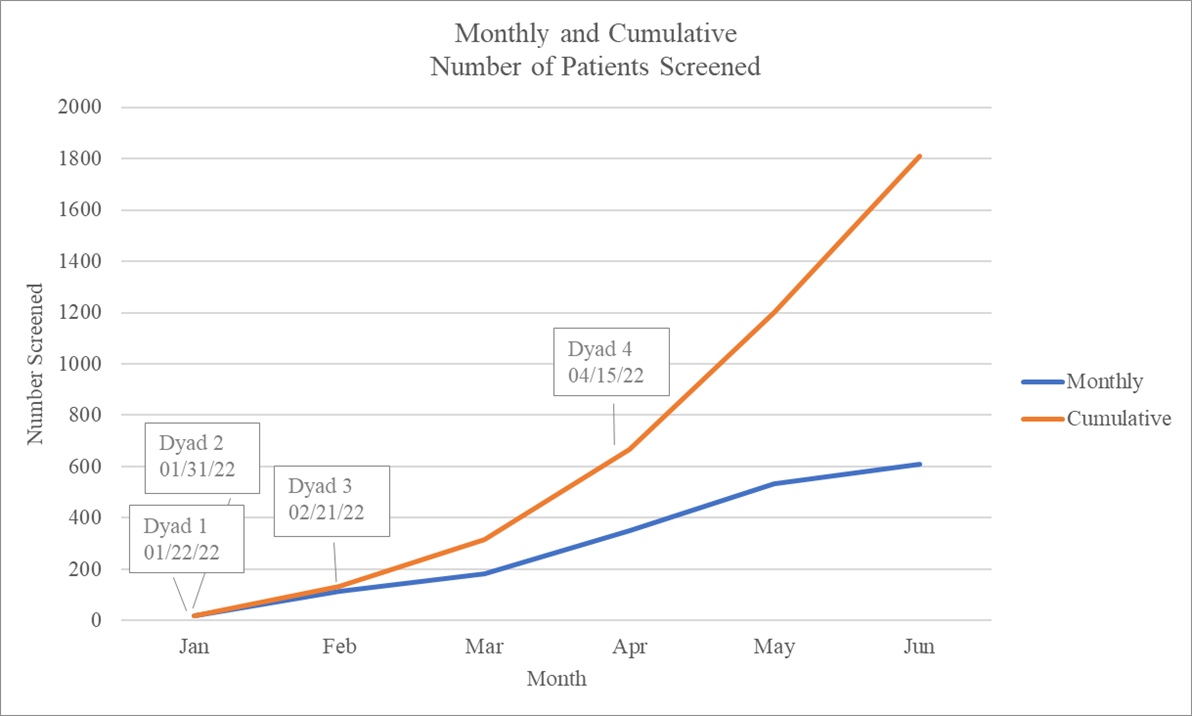
Results: For the 20 hub-spoke dyad months, the mean (range) composite feasibility score was 4.6 (4, 5). All study sites were retained during the pilot (100%, 8/8). Ninety percent (18/20) of monthly screening logs were completed on time. The eligibility error rate was 0.2% (3/1809). On-time data submission rate was 88.4% (84 of 95 case report forms). Eighty-five percent (17/20) of monthly sponsor site-dyad meetings were attended by both hub and spoke site staff.
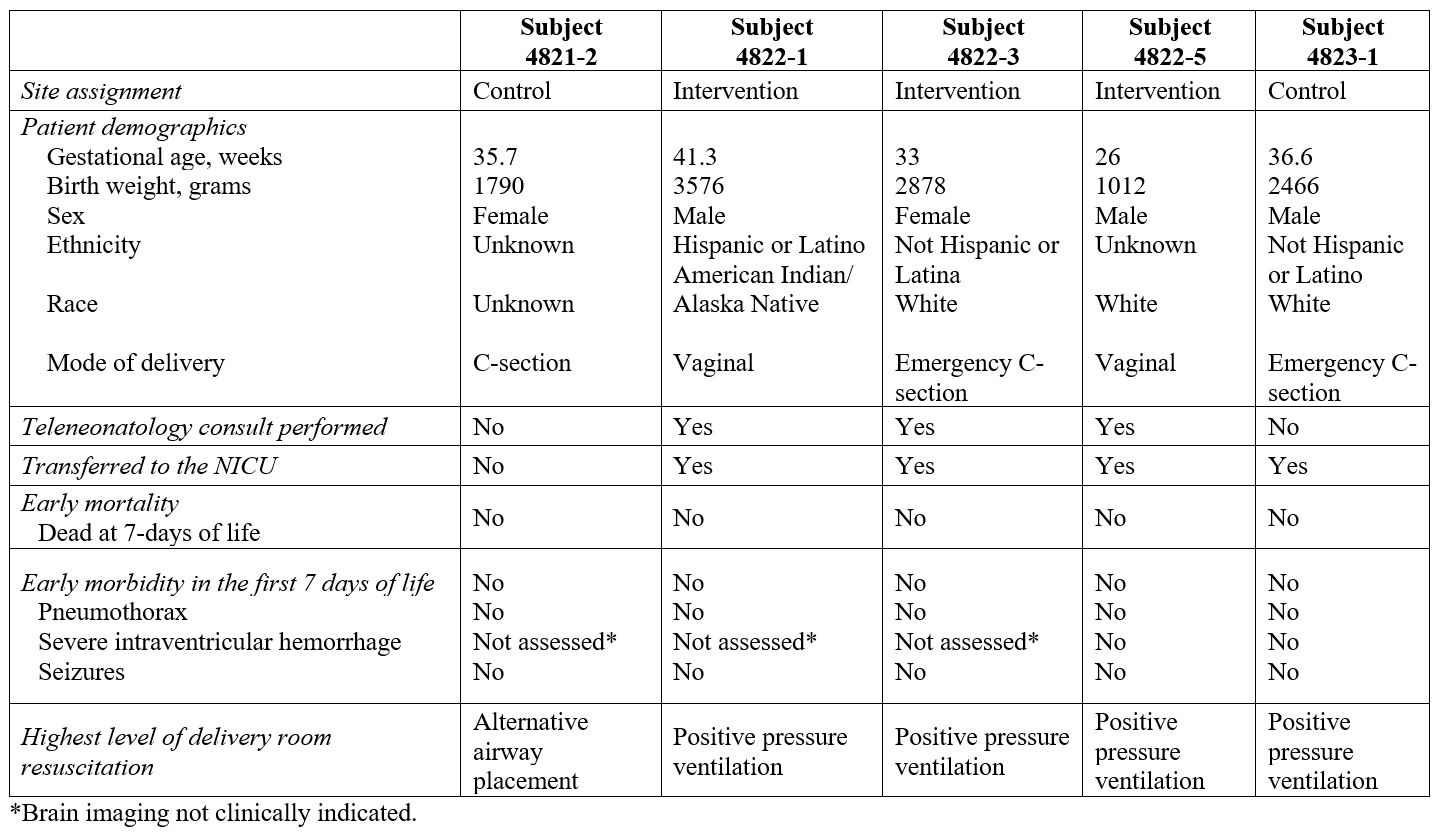
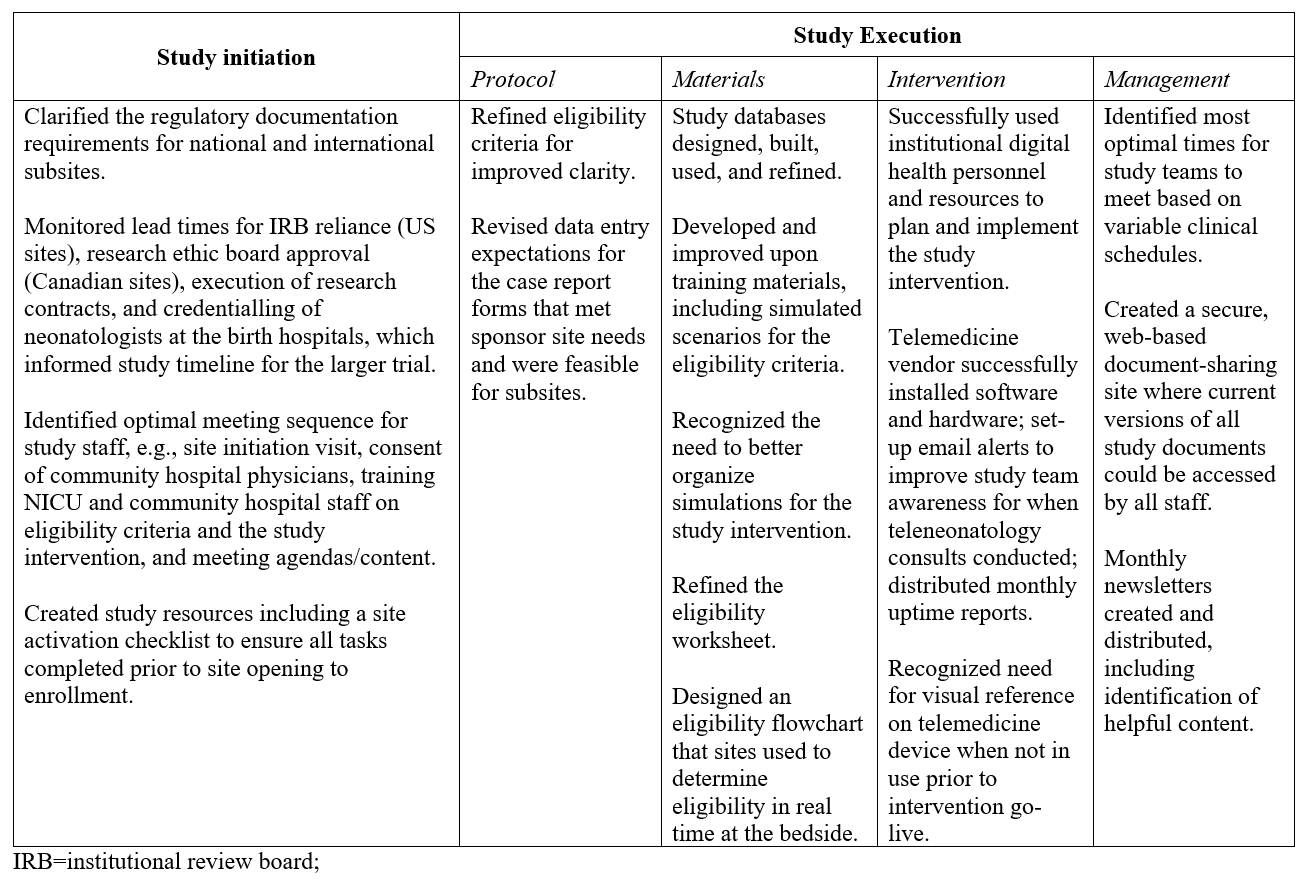
In total, 1809 newborns were screened for study eligibility at the four community hospital spoke sites (Figure 1). Five trial eligible patients were identified and enrolled, for an eligibility rate of 0.3% (95% CI 0.1%, 0.6%). Table 1 summarizes the subject characteristics and primary and secondary outcome assessments that will be used for the larger effectiveness trial. Achievements and learnings from the pilot trial are summarized in Table 2.
Conclusion(s): Using a composite feasibility score, we demonstrated that a multicenter teleneonatology clinical effectiveness trial is feasible. Learnings from the pilot study may improve the quality, efficiency, and likelihood of success of the main trial.