Neonatal Follow-up
NICU Follow Up and Neurodevelopment 6: The NICU Stay and Outcomes
49 - Association of Antenatal Steroid Exposure at ≤ 22 Weeks of Gestation with Survival without Severe Neurodevelopmental Impairment
Publication Number: 49.446
- SC
Sanjay Chawla, MD (he/him/his)
Professor of Pediatrics
Children's Hospital of Michigan
TROY, Michigan, United States
Presenting Author(s)
Background:
Antenatal steroids (ANS) given at 22 weeks’ gestation are associated with decreased mortality and short-term morbidities; however, data are lacking on neurodevelopmental outcome. Guidelines for providing antenatal steroids at 22 weeks vary.
Objective:
To compare rates of survival without severe neurodevelopmental impairment (sNDI), survival with sNDI, and death among infants born 220/7-236/7 weeks’ GA after exposure to ANS at ≤ 226/7 weeks’ GA versus no exposure to ANS.
Design/Methods:
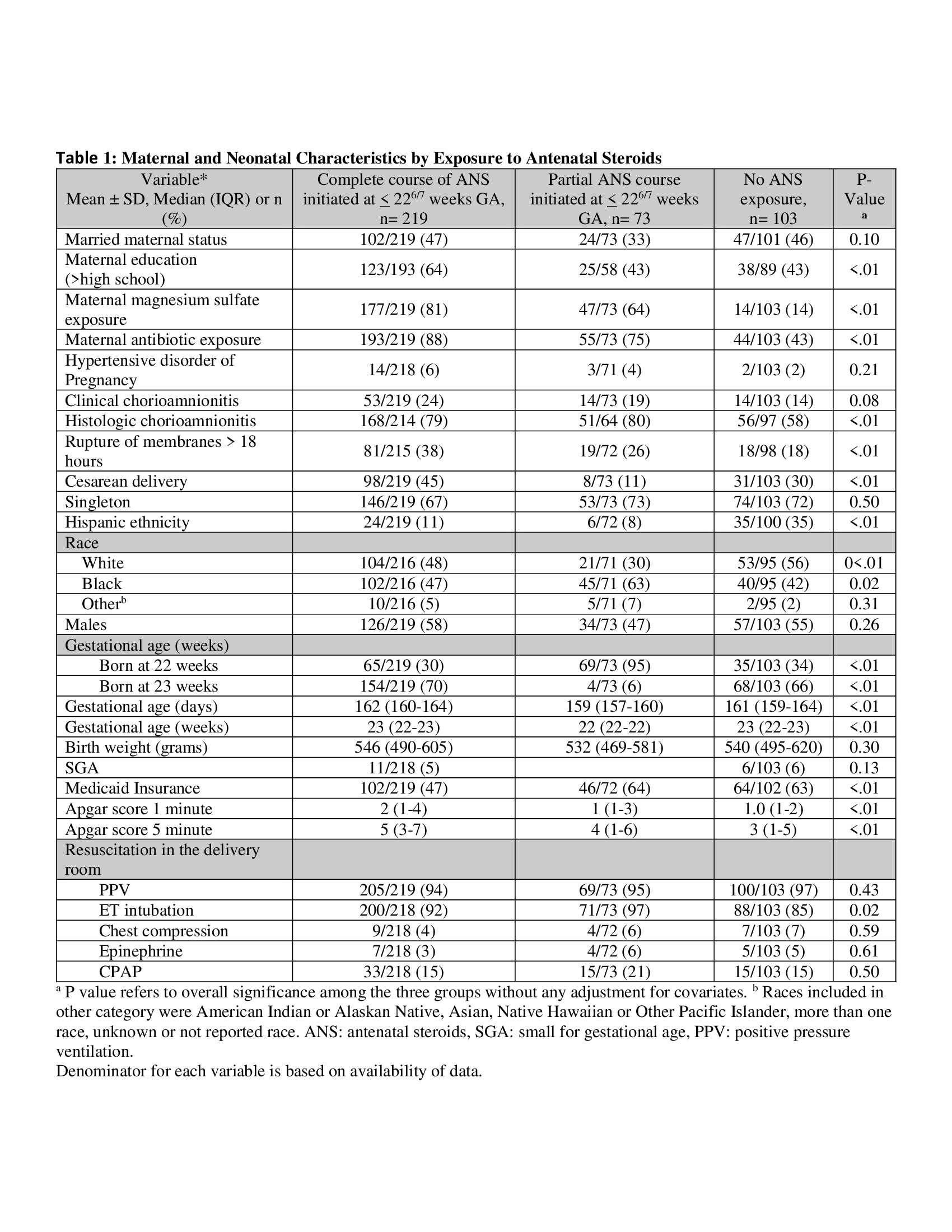
Infants were born at 220/7-236/7 weeks’ gestation between January 2016 and December 2019 at hospitals participating in the National Institute of Child Health and Human Development Neonatal Research Network. Infants who did not receive intensive care and infants with ANS exposure after 226/7 weeks’ GA were excluded. Primary outcome was a three-level polytomous outcome including survival without sNDI, survival with sNDI, or death. sNDI was defined as ³ 1 of the following: bilateral blindness, deafness, Gross Motor Function Level 4 or 5, motor and/or cognitive composite score < 70 assessed using Bayley Scales of Infant and Toddler Development III at 18-26 months’ corrected age. Infants were classified as having no, partial (1 dose) or complete (2 doses) exposure to ANS. The association of ANS exposure and outcomes was evaluated using Generalized linear mixed models for multinomial and binary outcomes, adjusting for GA, sex, race, Medicaid insurance, small for gestation, mode of delivery, multiple birth, prolonged rupture of membranes, birth year, antenatal magnesium sulfate, and birth hospital as a random effect.
Results:
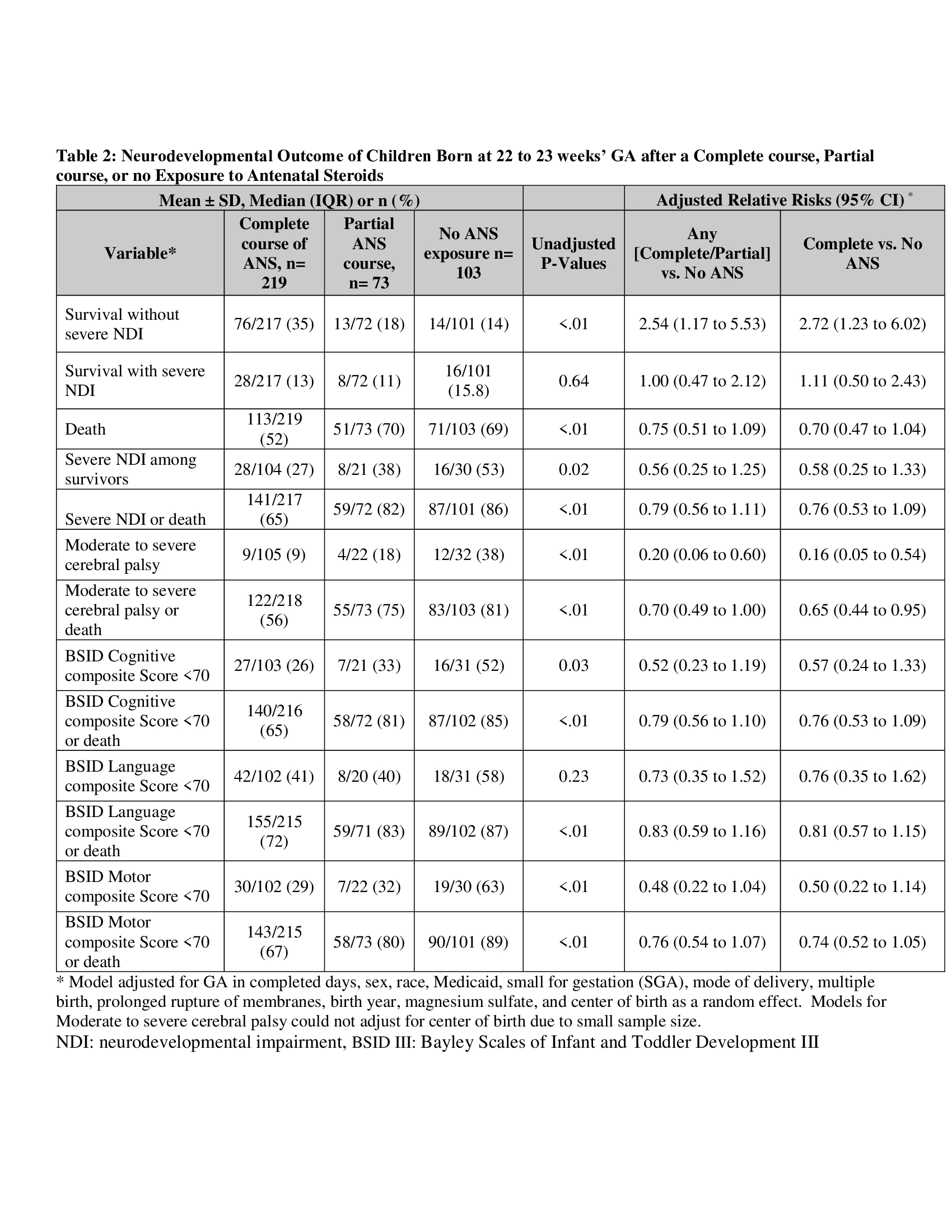
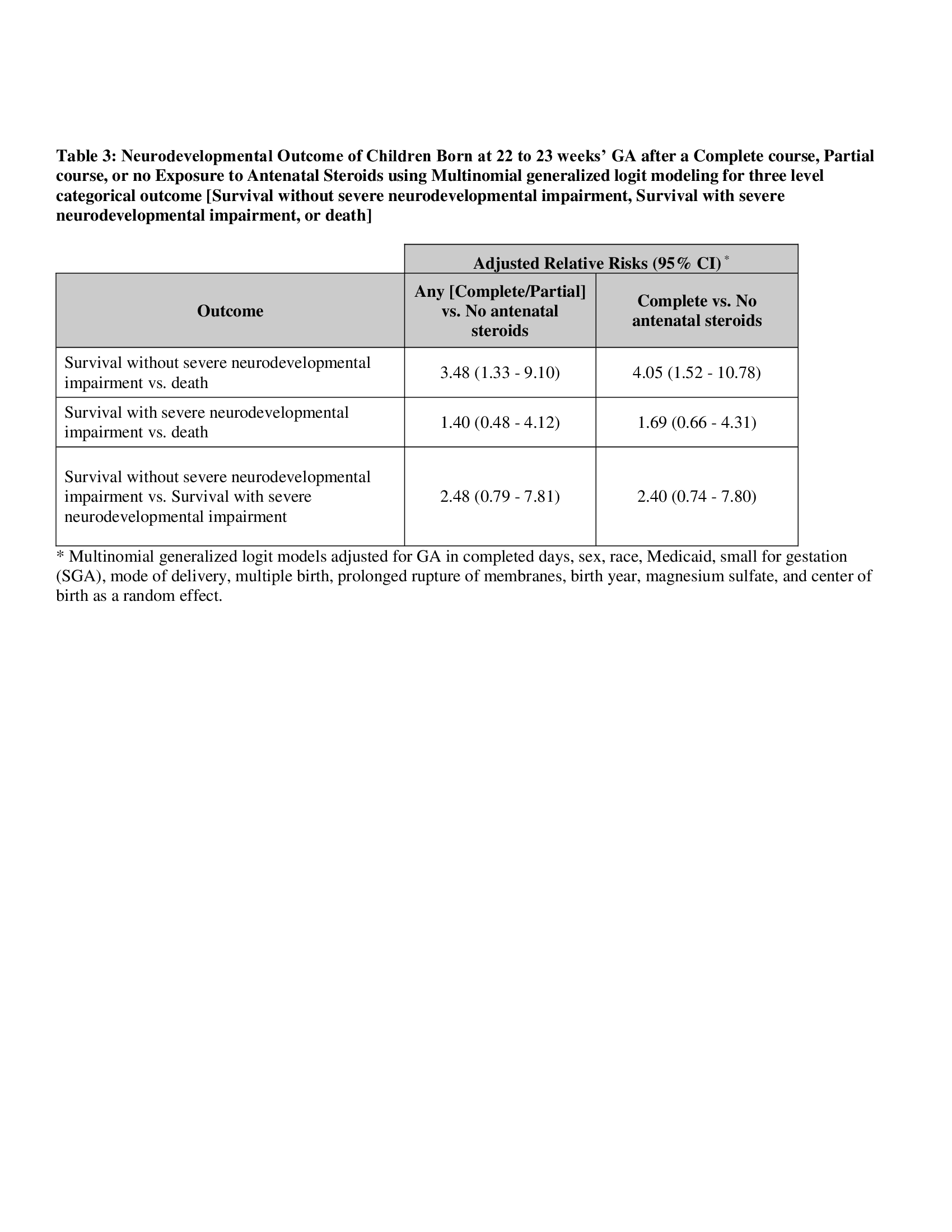
431 infants met the inclusion criteria; 14 had incomplete follow-up, 22 were lost to follow-up. Of the remaining 395 infants, 26.1% (n=103) were born with no ANS, 18.5% (n=73) with partial ANS, and 55.4% (n=219) with complete ANS exposure (Table 1). Among infants exposed to complete ANS, 35% survived without severe NDI, compared to 18% with partial ANS and 14% with no ANS (Table 2). The adjusted relative risk of survival without sNDI was significantly higher in infants born after any ANS exposure compared to those without ANS (aRR 2.54 [95% CI 1.17-5.53]). In the multi-level model, survival without sNDI vs. death was significantly higher for any ANS vs. no ANS group (aRR 3.48 [95% CI 1.33-9.10]), with no difference in survival with sNDI vs. survival without sNDI (aRR 2.48 [95% CI 0.70-7.81]) (Table 3).
Conclusion(s):
ANS administered to women at risk of imminent preterm birth at ≤ 226/7 weeks may be associated with a higher rate of survival without severe NDI but confounding cannot be excluded.