Neonatal Neurology: Pre-Clinical Research
Neonatal Neurology 8: Preclinical 2
17 - The complement-inflammasome axis mediates neuronal pyroptosis in neonatal hypoxic-ischemic encephalopathy
Publication Number: 17.434
- AS
Angela Saadat, PhD, MS
Laboratory manager
Eastern Virginia Medical School
Norfolk, Virginia, United States
Presenting Author(s)
Background:
There is an urgent need for new mechanistic insights into neonatal hypoxic-ischemic encephalopathy (HIE). The complement system plays critical homeostatic roles in the developing brain. However, complement dysregulation in HIE generates the potent anaphylatoxin C5a, mediating its effects via C5aR1 receptor signaling, culminating in the invasion of the CNS by pro-inflammatory cells and mediators such as Il-1β, which propagate brain injury. The NOD-like receptor family pyrin domain-containing-3 (NLRP3) inflammasome is a multiprotein complex that has been shown to induce neuronal pyroptosis by activation of caspase-1. Increased mRNA levels of NLRP3 correlate well with the severity of HIE.
Objective:
To test a complement-inflammasome axis in neonatal HIE, where C5a-C5aR interaction drives NLRP3 activation and downstream Il-1β production (Fig. 1).
Design/Methods:
Primary cortical neurons were subjected to oxygen-glucose deprivation (OGD), and subsequently treated with C5a or a combination of C5a+PMX205, a potent inhibitor of the C5a-C5aR interaction. Neuronal death was compared between groups. Term equivalent rat pups were subjected to the Vannucci model of neonatal HIE. A subset was treated with a single dose of PMX205, and rats were harvested 72h after injury. H&E staining was performed on paraffin-embedded brain sections to compare structural differences between groups. Immunofluorescence was performed to measure differences in expression of inflammasome markers between groups.
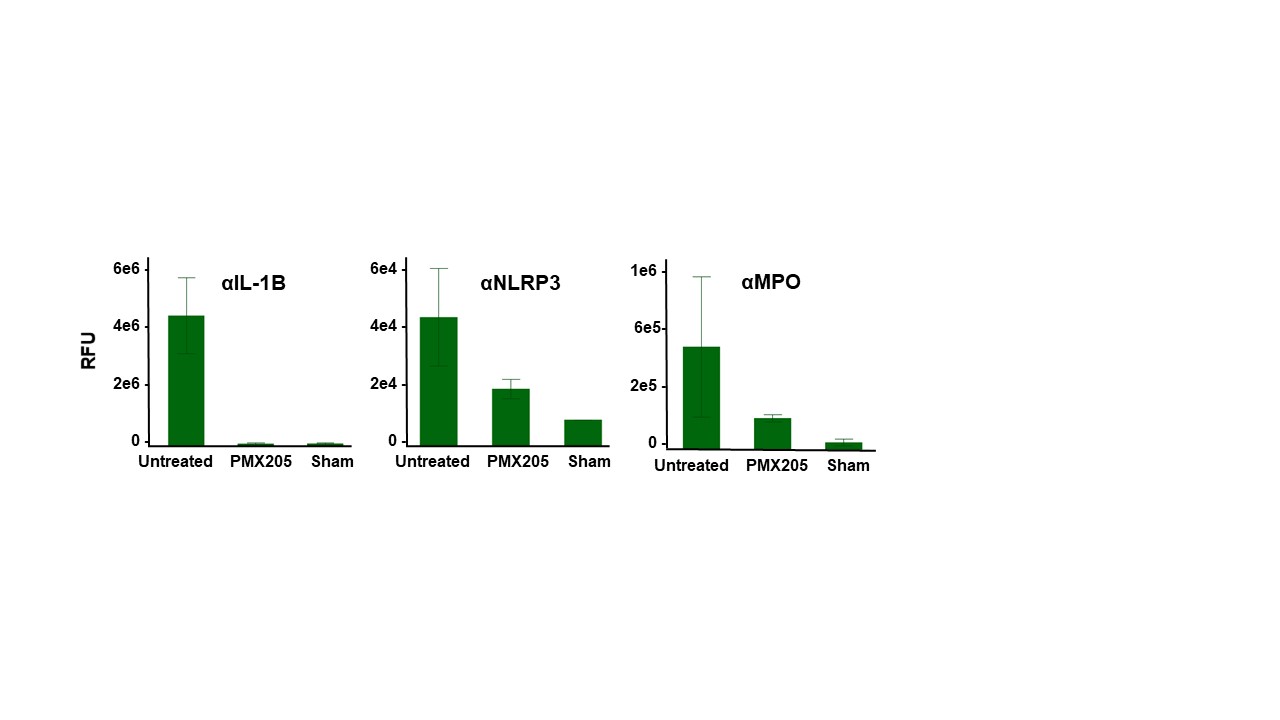
Results:
Treatment with PMX205 results in a significant decrease in cortical loss when compared to untreated controls in a rat model of neonatal HIE (Fig 2 A,B). Primary cortical rat neurons subjected to OGD followed by treatment with C5a resulted in significantly greater neuronal death compared to untreated neurons, and inhibition of C5a-C5aR1 signaling with PMX205 rescued neuronal death to levels comparable to untreated controls (Fig. 2C). Finally, neonatal rats subjected to hypoxia-ischemia demonstrate increased expression of inflammasome markers IL-1β, NLRP3 and myeloperoxidase in the brain. Treatment with PMX205 decreases their expression to sham levels (Fig. 3).
Conclusion(s):
These results reveal a potential new pathway perpetuating brain injury in neonatal HIE, providing a missing piece of the neuroinflammation puzzle. Modulating the complement-inflammasome axis may provide a viable therapeutic to mitigate reperfusion injury in HIE. Future steps include measuring inflammasome activation and neuronal pyroptosis in both in vitro and in vivo models of C5aR1 ablation.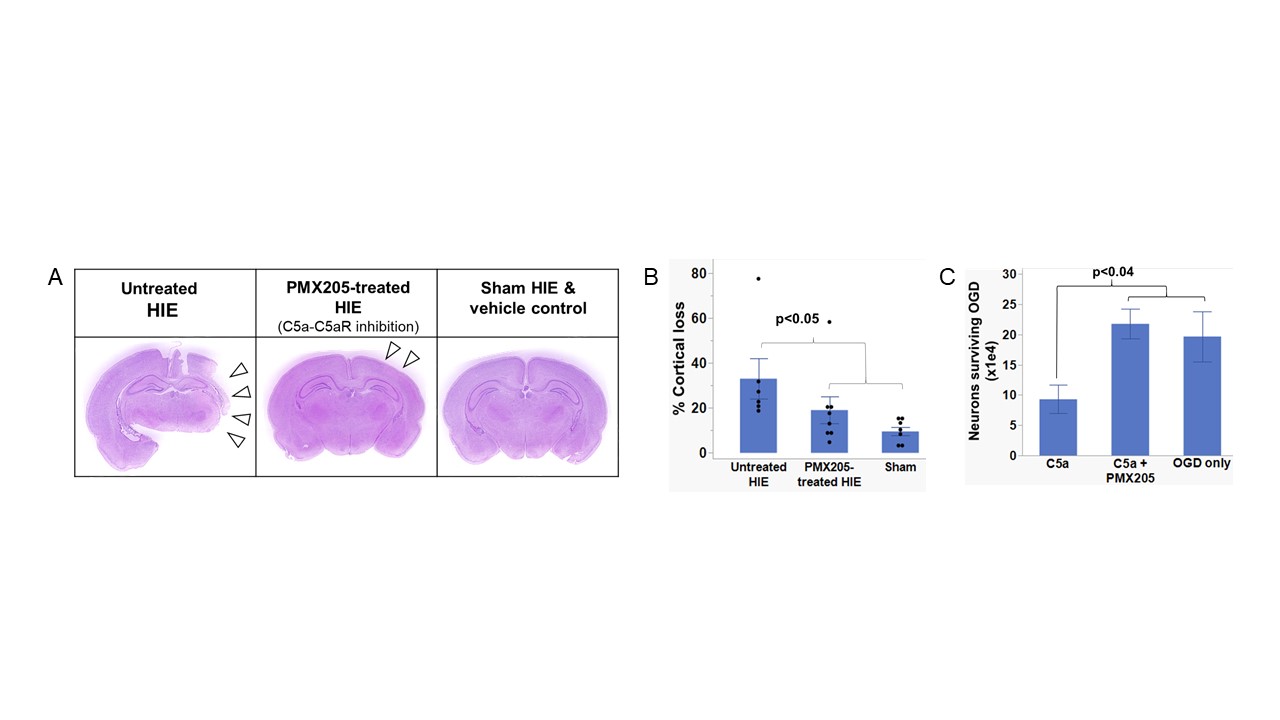
.jpg)
