Quality Improvement/Patient Safety: Subspecialty-specific QI: see specialties above
QI 6: Primary & Subspecialty Outpatient QI Group 1
252 - An Initiative to Standardize an Approach to Chronic Kidney Disease Patients with Growth Impairment
Publication Number: 252.452

Leyat Tal, MD (she/her/hers)
Pediatric Nephrology
Texas Children's Hospital
Houston, Texas, United States
Presenting Author(s)
Background:
Short stature associated with chronic kidney disease (CKD) is multifactorial and under recognized. Short stature not only affects mental well-being, but also has health consequences such as increased hospitalization, neurological impairment and a higher risk of death. While one-third of children with CKD have growth failure, growth hormone (GH) therapy is prescribed in less than 25% of patients with short stature. Barriers to using GH include daily injections, concern for side effects, and cost.
Objective:
- Develop a systematic approach for identifying patients with CKD 3-5 and short stature who are growth hormone eligible
- Increase percentage of patients with anthropometrics evaluation
- Increase percentage of patients initiated on GH
Design/Methods:
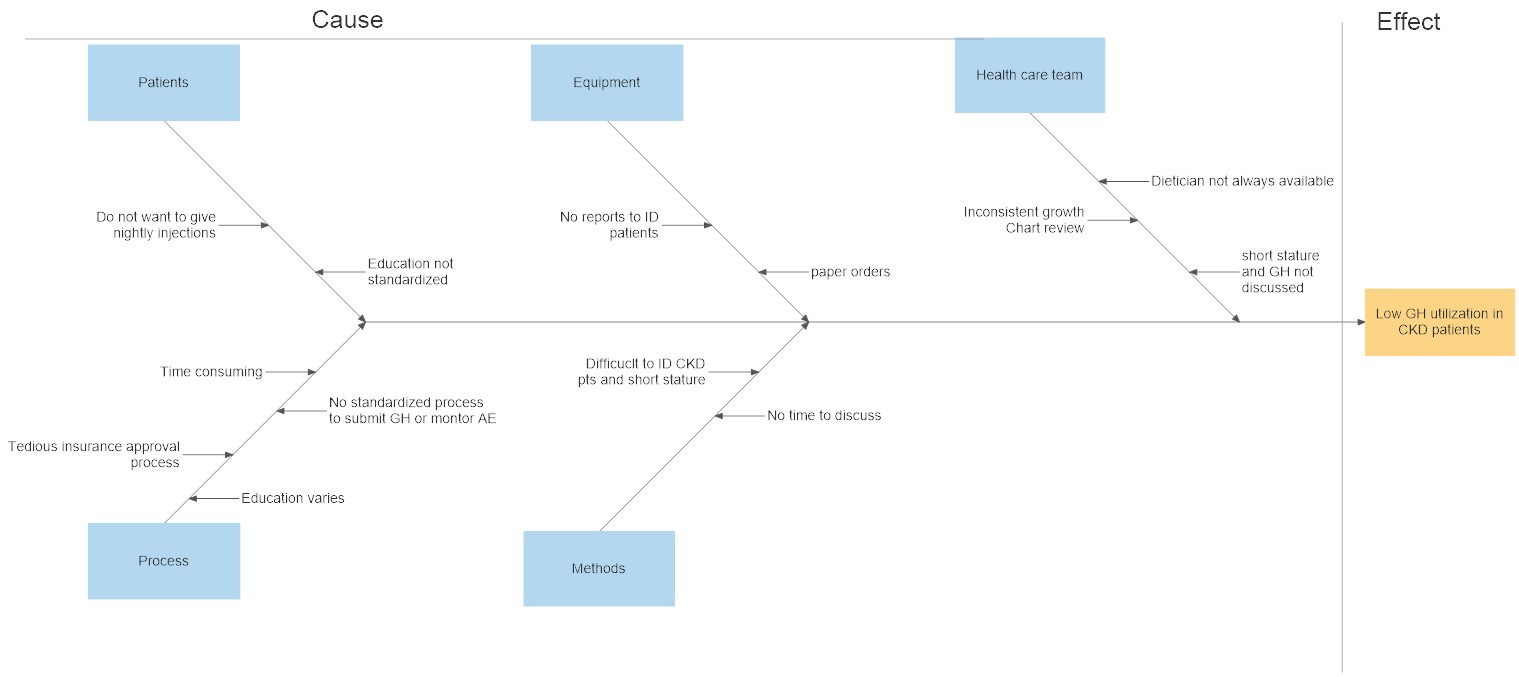
Prospective quality improvement project was conducted at Texas Children’s Hospital nephrology clinic. Development of a process flow diagram for a standardized approach for identifying and evaluating patients for GH. A monthly report was generated to include all patients who were 1-17 years old, CKD 3-5, height z-score less than -1.5, and an upcoming renal visit. Dieticians evaluated these patients’ anthropometrics and discuss GH with the provider and/or family. A fish bone diagram was done to review potential factors related to low GH utilization. Identifying patient/family education as a common factor, an education handout was created. The pilot study was implemented from June 1 to December 31, 2022.
Results:
51/128 patients with CKD 3-5 met criteria for short stature. 7 patients were already on GH therapy. 17/44 patients were deemed medically inappropriate for GH by their primary nephrologist at initial screening, with underlying genetic syndrome and renal transplant in last 1 year as the most common reasons. 27 patients had their anthropometric measurements reviewed in clinic by a dietitian. In consultation an additional 16 patients were not deemed medically suitable for GH. 50% of patients had not seen or had growth parameters reviewed by a dietitian in the last 6 months which improved to 100%. 11 patients were eligible for growth hormone. 55% of patients agreed to initiate GH. 5 patients who did not agree to GH, the most common reason was the fear of daily injections.
Conclusion(s): Through the development of a standardized approach we were able to identify all patients who were eligible for GH and provide standardized education on the benefit of GH use. Next steps include reviewing patient and physician barriers to GH usage and identifying CKD patients at risk for growth impairment with the ultimate goal of increasing GH utilization in CKD patients..jpg)