Neonatal Clinical Trials
Neonatal Clinical Trials 2
275 - Efficacy and Safety of Short-Course Antibiotic Therapy (72hrs) Compared to Standard Duration (5-7 Days) in Preterm Neonates with Culture-Negative Early-Onset Sepsis: A Randomized Control Trial
Publication Number: 275.428
- SN
Sushma Nangia, MBBS, MD, DM (she/her/hers)
Professor
Lady Hardinge Medical College & Kalawati Saran Children's Hospital
New Delhi, Delhi, India
Presenting Author(s)
Background:
Few studies have recently compared short-course therapy (ranging from 3-5 days) with standard antibiotic duration (5-7 days) in late preterm and term neonates with culture-negative early-onset neonatal sepsis. Infants born at earlier gestational ages (28-34 weeks) were either excluded or formed a small subset. Determining the exact duration of antibiotic therapy for culture-negative sepsis in preterm neonates is still debatable and has not been thoroughly researched.
Objective:
This open label randomized control trial compared the occurrence of treatment failure among preterm infants with symptomatic, culture negative, early onset neonatal sepsis, receiving short course antibiotic therapy (72 hours) or standard duration of antibiotic therapy (5-7 days).
Design/Methods:
Preterm infants with birth weight ≥1000 gms and/or gestation age ≥28 weeks, presenting with clinical symptoms suggestive of neonatal sepsis within 72 hours of life, received first line antibiotics as per the unit policy. After 72 hours of receiving antibiotics if the result of blood culture was negative and symptoms had resolved, the infants were randomly allocated to short course (72hrs) or standard course (5-7days) therapy groups. The main outcome was treatment failure defined as reappearance of symptoms of sepsis within 15 days after discontinuation of antibiotics warranting sepsis workup and antibiotic treatment. Infants with congenital anomalies, site specific infections, IEM, severe perinatal asphyxia or those undergoing surgery were not included.
Results:
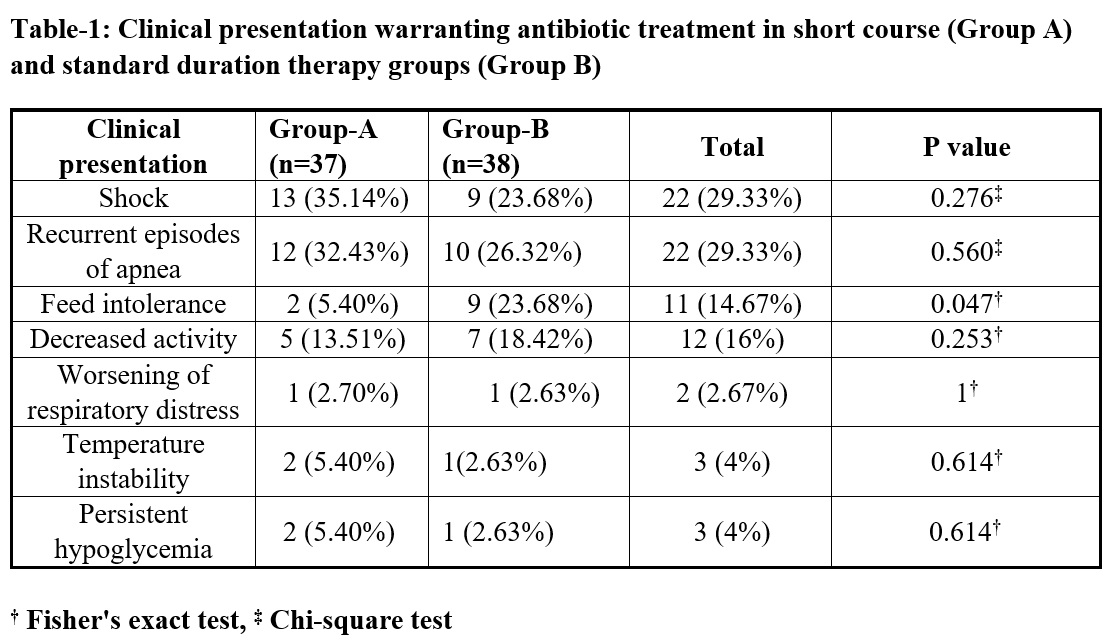
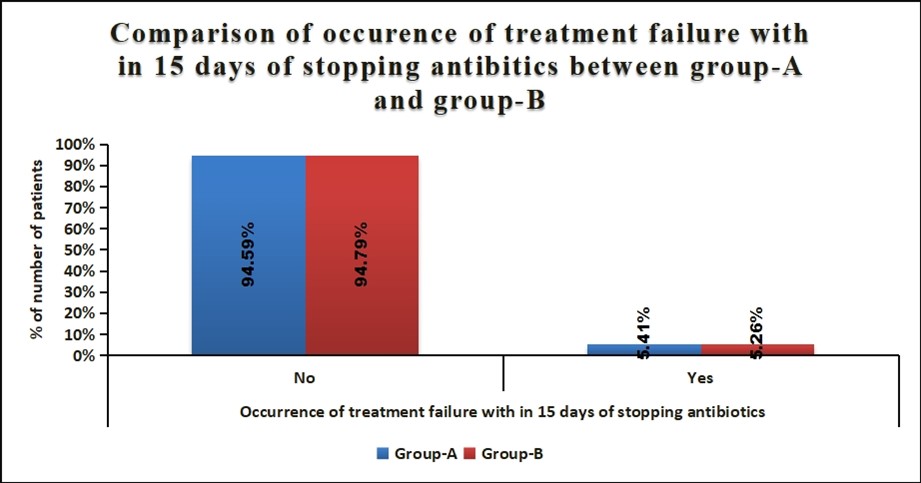
Seventy-five eligible neonates (mean GA 31+2 weeks, mean birthweight 1504+459 grams) with symptomatic early onset sepsis (Table 1) were randomized to receive either short or standard duration of antibiotic therapy. On admission to the NICU, 96% required respiratory support [(MV/NIV/CPAP/HHHFNC), (median duration 72 (48-144) hours)] and 36% received surfactant therapy with no difference between the two groups. No infant developed IVH, ROP or BPD but in the standard arm 2 babies developed NEC>stage 2b and 2 others required PRBC transfusion. Mean duration of hospital stay was comparable between groups being 22 and 24 days. Two infants each in short course and standard duration therapy had treatment failure (P=1) and one infant with NEC succumbed post-surgery in the standard arm.
Conclusion(s):
Occurrence of treatment failure among preterm symptomatic neonates with culture negative early onset sepsis did not differ between short course (72hours) and Standard duration (5to7days) of antibiotic therapy. No serious harm was observed with short course antibiotic regimen.