Neonatal Pulmonology
Neonatal Pulmonology 5: NICU Practices
157 - Battle of the Surfs: Comparing Calfactant and Poractant Alfa’s Outcomes in Premature Neonates
Publication Number: 157.437
- EF
Emilie M. Flament, DO (she/her/hers)
Resident
Prisma Health Greenville
Greenville, South Carolina, United States
Presenting Author(s)
Background: Natural exogenous surfactants are the leading treatment of neonatal respiratory distress syndrome (RDS). There are limited studies comparing patient outcomes from treatment of two of the most widely used formulations of surfactant.
Objective: To compare clinical outcomes of infants who received Calfactant (Infasurf) and Poractant Alfa (Curosurf) in the treatment of RDS.
Design/Methods:
This is a retrospective cohort study of the inborn neonates less than 34 weeks gestation at Greenville Memorial Hospital who received either Calfactant or Poractant Alfa for two six-month time periods. Neonates treated with multiple types of surfactants and those treated for meconium aspiration were excluded. Electronic medical records were reviewed for demographic data, time of surfactant administration, maternal betamethasone administration, as well as clinical outcomes including the incidence of mortality, pneumothorax, and chronic lung disease (CLD), and days of ventilation (invasive and noninvasive). Statistical analysis was performed using paired t-test and SPSS software.
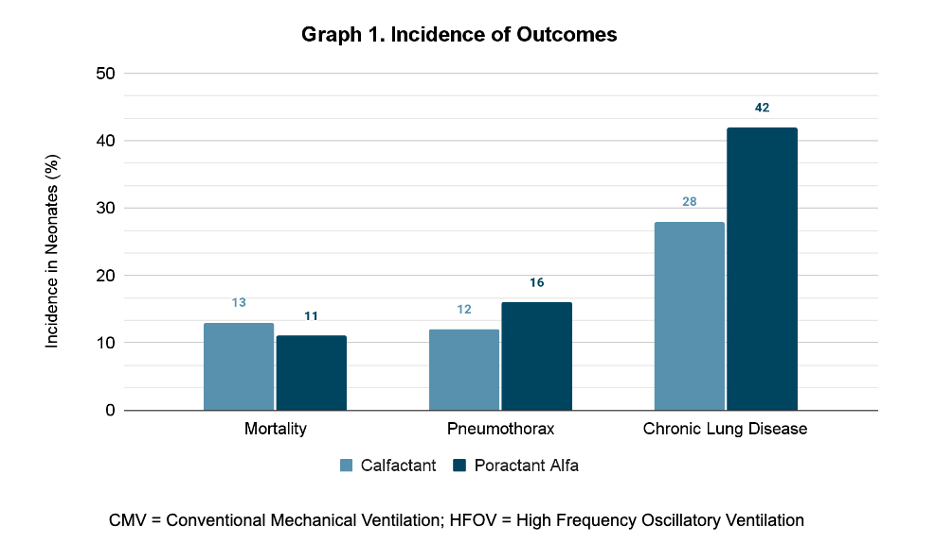
Results: 152 neonates (247 doses of surfactants) were reviewed. 78 infants received Calfactant and 74 infants received Poractant Alfa. There was no statistical difference in the demographics of the two groups, except for birth weight and antenatal steroids (Table 1). The rates of mortality, pneumothorax, and CLD did not differ significantly between the Calfactant and Poractant Alfa groups: mortality rates 13% and 11%, respectively; pneumothorax rates 12% and 16%, respectively; CLD rates 28% and 42%, respectively (Graph 1). There were also no significant differences in days of invasive or non-invasive ventilation (Graph 2).
Conclusion(s): There was no significant statistical difference in the incidence of mortality, pneumothorax, CLD, nor in the days requiring non-invasive ventilation and invasive ventilation in the Calfactant and Poractant Alfa groups. This study adds to the literature more evidence that there is no significant difference between currently available brands of exogenous surfactants..png)
.png)
