Neonatal Quality Improvement
Neonatal Quality Improvement 6
210 - Prevention of Problematic Metabolic Acidosis in Very Low Birth Weight Infants: A Quality Improvement Initiative
Monday, May 1, 2023
9:30 AM - 11:30 AM ET
Poster Number: 210
Publication Number: 210.44
Publication Number: 210.44
Christopher C. Stryker, Goryeb Children's Hospital, Morristown, NJ, United States; Ruth Snyder, MidAtlantic Neonatology Associates, Somerset, NJ, United States; Christine A. Robinson, Morristown Medical Center, Morristown, NJ, United States; Christin Aikey, Goryeb Children's Hospital, Morristown, NJ, United States; Colleen Quinn, Morristown Medical Center, Montclair, NJ, United States; Melissa Neavear, Atlantic Health Systems, Hoboken, NJ, United States; Ian J. Griffin, MANA, Morristown, NJ, United States

Christopher C. Stryker, MD (he/him/his)
Attending Neonatologist
MidAtlantic Neonatology Associates/Atlantic Health System/Goryeb Children's Hospital
Morristown, New Jersey, United States
Presenting Author(s)
Background: VLBW infants (birth weight < 1500g) are predisposed to developing metabolic acidosis in early life due to increased acid production associated with clinical conditions that cause tissue hypoxia and an inability to effectively excrete acid due to renal immaturity. Metabolic acidosis may adversely affect organ function and growth. Nutritional practices, including provision of parenteral electrolyte and nutrition (TPN) solutions, may impact acid-base balance in preterm infants.
Objective: Reduce the incidence of problematic metabolic acidosis (MA) in VLBW infants by 25% in 12 months, while avoiding adverse effects on short-term growth.
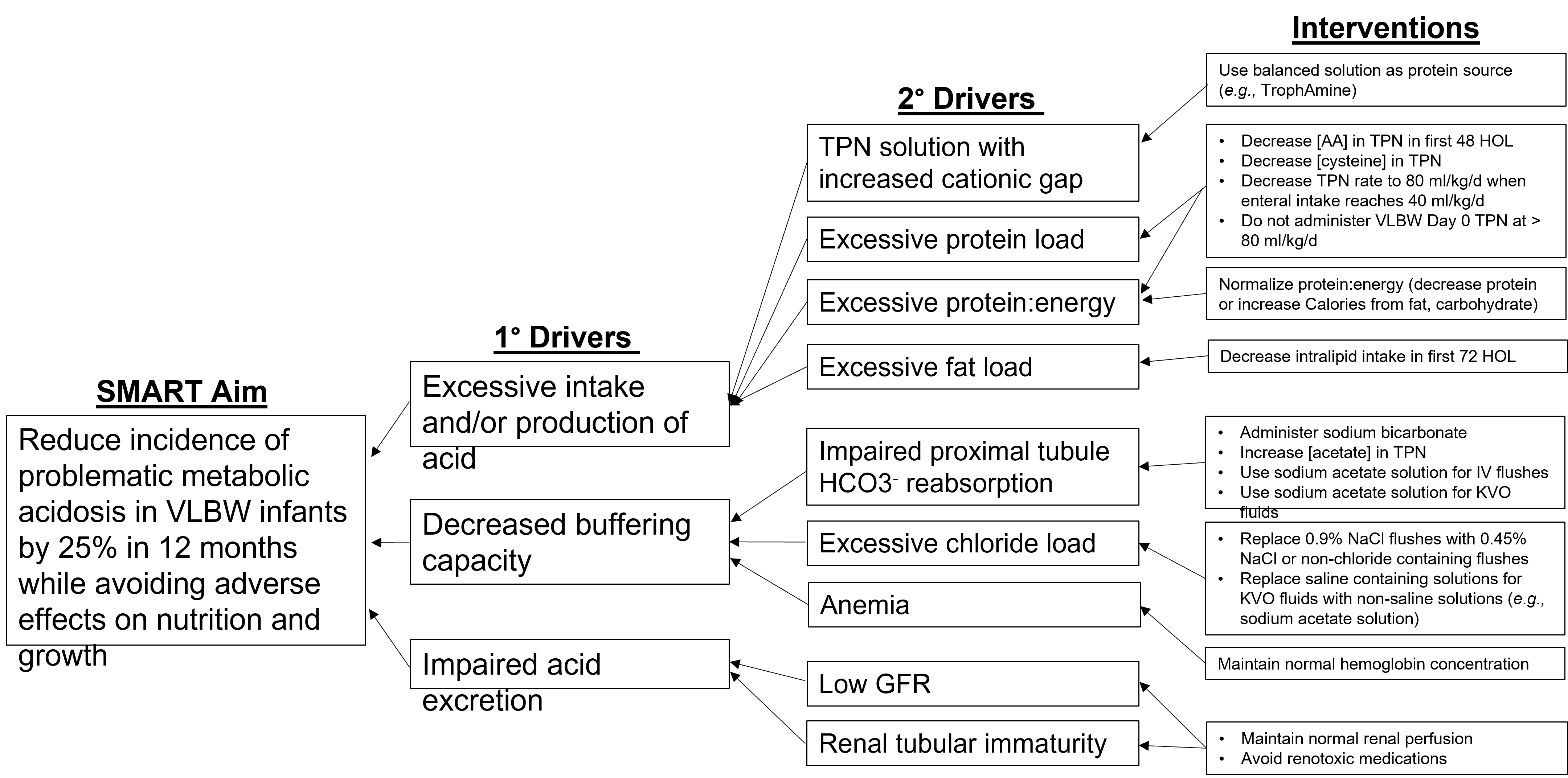
Design/Methods: MA was defined as BE ≤ -10 mmol/L, or if blood gas unavailable, CO2 < 15 mmol/L, with a normal anion gap (< 16 mmol/L) and lactate < 5 mmol/L occurring between days 2 and 5 of life. All VLBW infants born at Morristown Medical Center who survived > 5 days were included. Key drivers contributing to metabolic acidosis were identified; interventions were devised to decrease chloride and increase acetate administration and optimize the ratio of protein to energy intake (Fig 1). Specific interventions were as follows: Limit VLBW Day 0 TPN rate to 80 mL/kg/d; decrease TPN rate to 80 mL/kg/d when enteral feeds reach 40 mL/kg/d; order 0.45% sodium acetate solution for UAC KVO fluids. The primary outcome measure was the proportion of infants with MA per cohort of 7 consecutively born VLBW infants. Balancing measures included time to regain birth weight (BW) and extrauterine growth restriction (EUGR). Compliance with interventions was monitored.
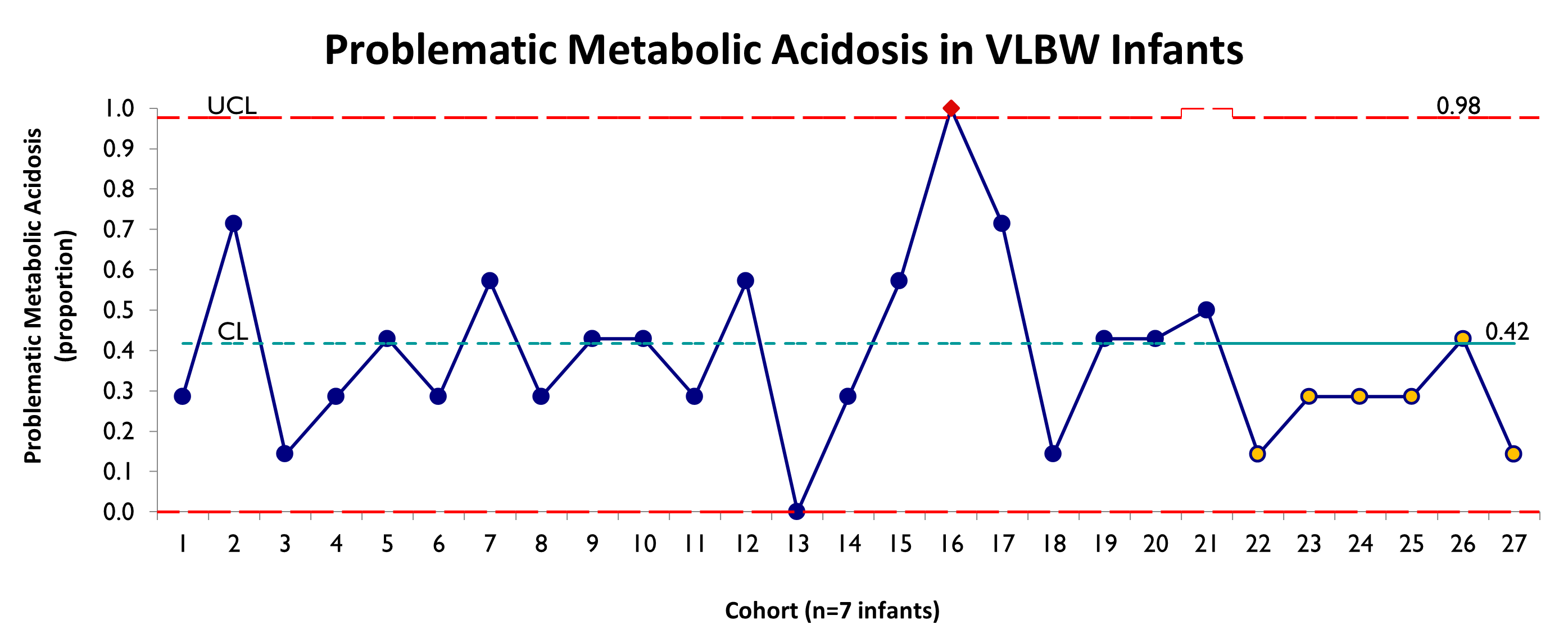
Results: In a historical cohort, 42% of VLBW infants developed MA (n=82). In comparison, 26% of infants in the intervention cohort developed MA (n=44). There was a trend toward decreased MA, but no special cause variation (Fig 2). There were no differences between the historical and intervention groups in time to regain BW (9.1 d vs. 8.8 d, respectively; p=0.72) or EUGR (ΔZ >1 at 36 wks CGA 22.5% vs. 14.5%, respectively; p=0.45). Six protocol violations were identified; compliance with all interventions for each infant was 86%.
Conclusion(s): Using QI methodology, we devised and implemented interventions to decrease MA in VLBW infants. While there was a trend toward decreased MA, there was no special cause variation within the study period. Interventions did not adversely affect growth. Further interventions have since been implemented, including a decrease in the amino acid and cysteine concentrations in the day 0 VLBW TPN formulation. Data collection and analysis are on-going.