Infectious Diseases
Infectious Diseases 5
435 - Impact of Acyclovir Ordering Restriction on Neonatal HSV Evaluations
Publication Number: 435.419

Cindy Nguyen, MD (she/her/hers)
Pediatric Hospital Medicine Fellow
University of Arkansas for Medical Sciences
Little Rock, Arkansas, United States
Presenting Author(s)
Background:
Neonatal herpes simplex virus (HSV) causes significant morbidity and mortality. Early treatment with acyclovir is critical. Evaluation of suspected neonatal HSV infections includes HSV polymerase chain reactions (PCRs) or cultures from the serum, cerebral spinal fluid (CSF), and mucosal sites as well as serum alanine aminotransferase (ALT). However, adherence to these testing recommendations vary.
Objective: Evaluate impact of acyclovir prescribing restriction changes on evaluation and clinical outcomes of neonatal HSV.
Design/Methods:
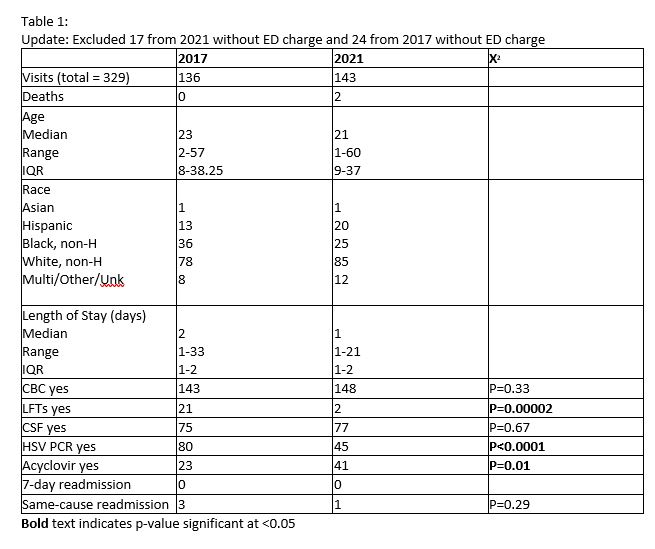
This is a retrospective analysis of infants, aged 0-60 days, admitted to a tertiary pediatric teaching hospital with suspected HSV during different periods of acyclovir restriction. Infants were identified in the PHIS database with a diagnosis of fever of newborn, fever unspecified, neonatal sepsis, meningitis, HSV meningitis, meningoencephalitis, hypothermia in neonate, congenital herpes viral infections, herpes viral ocular disease, disseminated herpes viral disease, and herpes viral infection NOS. A "complete" workup was defined as a complete blood count, liver function tests, CSF analysis, and HSV PCRs. Rates of these individual tests, as well as rates of acyclovir administration, readmission, and deaths were analyzed and compared using χ2.
Results:
There were a total of 329 infants included in this study (136 in 2017 – ID approval period, and 143 in 2021 – restriction released). The age, race, and ethnicities of both groups were similar (Table 1). In the ID approval period, neonates with suspected HSV were significantly more likely to have both HSV PCR and ALT evaluated compared to those in the period without acyclovir restriction (P< 0.0001 and P< 0.00002, respectively). There was no statistically significant difference in rates of readmission (ID approval period, 3; Restriction released period, 1) or death (ID approval period, 0; restriction released period 2).
Conclusion(s):
At this tertiary pediatric hospital, the restriction of acyclovir usage increased the number of infants with a “complete” workup for neonatal HSV but did not significantly impact the outcome. Importantly, releasing the requirement for infectious disease approval of acyclovir use in infants has not resulted in a statistically significant increase in readmissions or death in these infants. While antimicrobial restriction can be a valuable tool in optimizing patient care, the impact of restriction or approval programs on clinical outcomes must be followed closely to ensure that the goals of the program are being met without unintended consequences such as delays in care.