Neonatal Infectious Diseases/Immunology
Neonatal Infectious Diseases/Immunology 6
469 - Risk Phenotype Association with Early-Onset Sepsis in Preterm Infants
Monday, May 1, 2023
9:30 AM - 11:30 AM ET
Poster Number: 469
Publication Number: 469.432
Publication Number: 469.432
Sarah A.. Coggins, Childrens Hospital of Philadelphia, Philadelphia, PA, United States; Sagori Mukhopadhyay, Childrens Hospital of Philadelphia, Philadelphia, PA, United States; Karen M. Puopolo, Perelman School of Medicine at the University of Pennsylvania, Philadelphia, PA, United States
- SC
Sarah A. Coggins, MD
Neonatologist
Childrens Hospital of Philadelphia
Philadelphia, Pennsylvania, United States
Presenting Author(s)
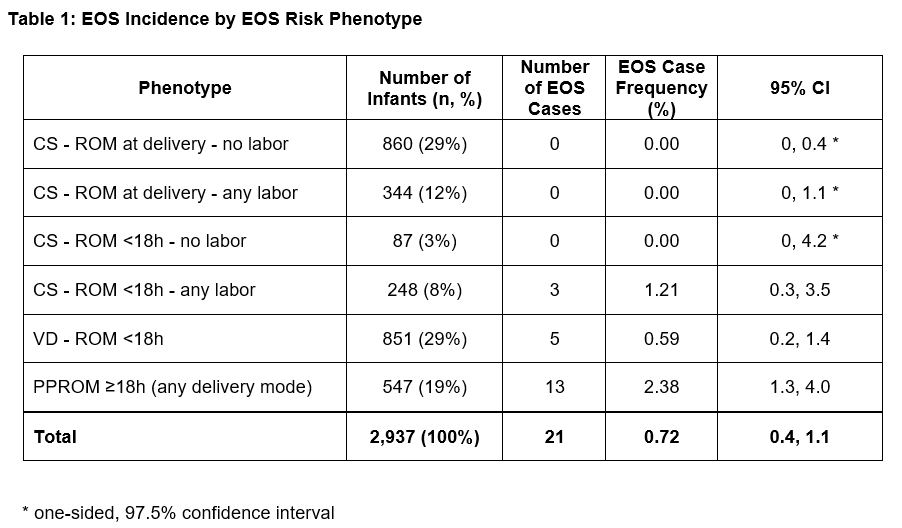
Background: Neonatal early-onset sepsis (EOS) disproportionately affects preterm infants, leading to widespread empiric antibiotic use at birth. While antibiotics are administered to protect infants from severe infection, most infants are ultimately uninfected, with evidence suggesting adverse neonatal outcomes associated with early antibiotic exposures. Aside from an AAP-endorsed “low-risk EOS” birth phenotype (birth via Cesarean section (CS), no labor, rupture of membranes (ROM) at delivery), a scenario in which empiric antibiotics may be reasonably deferred, there are no comprehensive data-driven approaches to preterm EOS risk stratification.
Objective: To assess EOS incidence among prespecified EOS risk phenotypes based on delivery characteristics.
Design/Methods: Retrospective cohort study of preterm infants born < 35 weeks gestation at four Philadelphia-area perinatal centers during 2017-2021. We constructed EOS risk phenotypes based on variables associated with EOS pathogenesis (presence of labor, ROM duration, delivery mode), and a priori created a phenotype including all infants born following prolonged preterm ROM (PPROM), due to its hypothesized association with EOS risk regardless of delivery mode or labor. EOS was defined as pathogen isolation from blood or cerebrospinal fluid in the first 72 hours after birth, excluding common commensals. The primary outcome was EOS incidence within the overall cohort and within each phenotype.
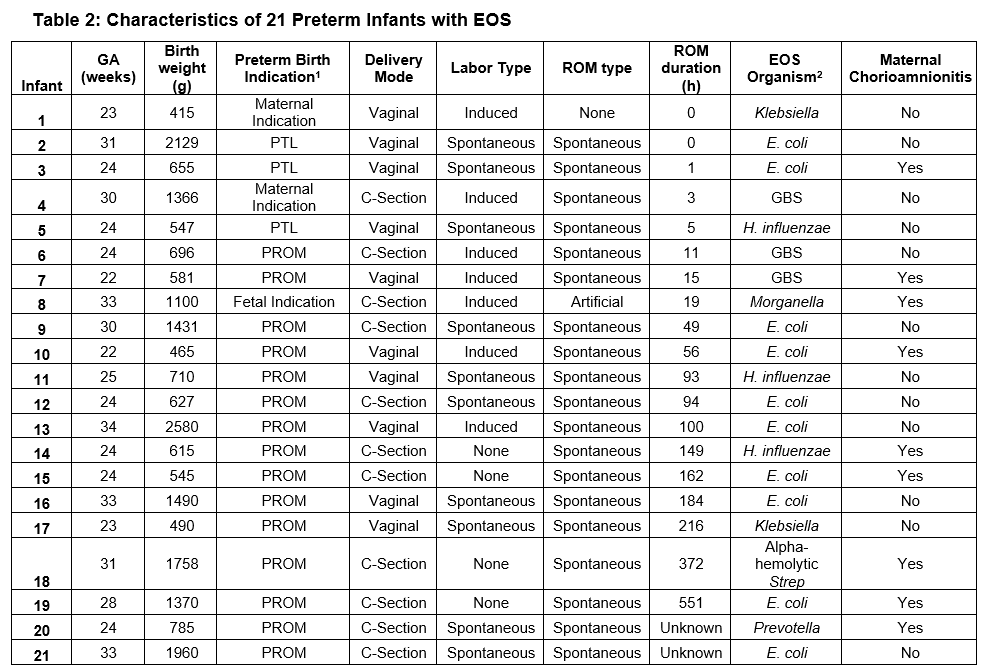
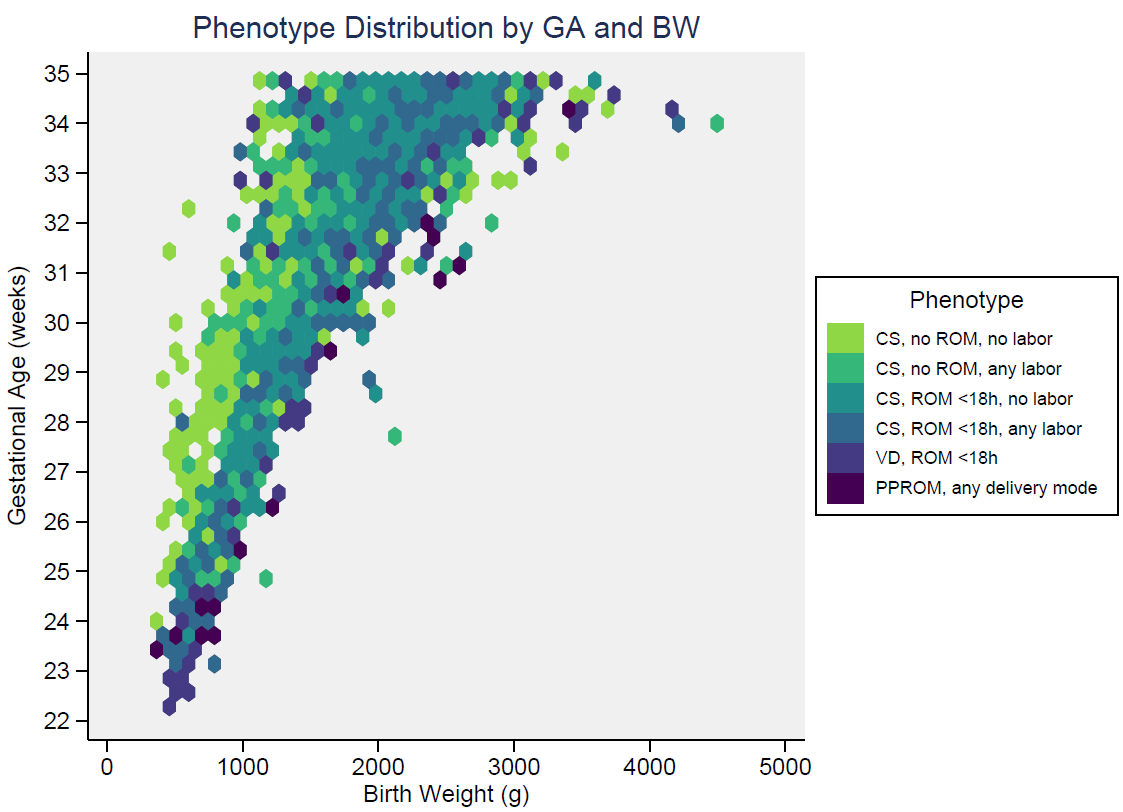
Results: There were 2937 infants included in the analysis, with median gestational age (GA) 33 weeks and birth weight (BW) 1795 grams. Among these, 1907 (65%) infants received an EOS evaluation (blood culture and antibiotics) at birth. Six phenotypes were identified, with EOS distribution across the cohort shown in Table 1. 29% of infants met the AAP “low-risk” phenotype designation (CS, no labor, ROM at delivery). Figure 1 shows phenotype distribution by GA and BW. 21 infants had EOS (0.7%). The majority of EOS cases (13/21, 62%) occurred in the setting of PPROM, with a phenotype EOS frequency of 2.4% (Table 1). There were no EOS cases among infants born by CS without ROM (with or without labor), nor via CS with ROM < 18 hours without labor. Table 2 presents characteristics of infants with EOS.
Conclusion(s): Within a cohort of almost 3,000 preterm infants, we identified differential EOS incidence by EOS risk phenotype. These phenotypes may inform EOS risk stratification and antibiotic stewardship in preterm infants, but require validation in larger cohorts to inform robust population estimates of EOS incidence.