Infectious Diseases
Infectious Diseases 4
416 - Are We Dosing Correctly? Pharmacokinetics of Cefepime in Critically Ill Pediatric Patients
Publication Number: 416.418

Stephanie L. Rolsma, MD, PhD (she/her/hers)
Assistant Professor
Vanderbilt University Medical Center
Nashville, Tennessee, United States
Presenting Author(s)
Background:
Dosing guidelines of cefepime are well defined in healthy pediatric and adult populations but have not been extensively studied in critically ill pediatric patients, particularly those receiving ECMO and CRRT. Patients who are critically ill may have significant alterations in antibiotic pharmacokinetics (PK) and pharmacodynamics (PD) due to many factors. Receipt of ECMO or CRRT may further alter PK/PD due to changes in clearance and volume of distribution and the depletion of plasma proteins.
Objective:
Since critically ill pediatric patients are likely to receive multiple courses of antimicrobials, optimization of drug dosing and delivery is critically important for individual patient outcomes. This study will test the hypothesis that the PK of beta-lactam antibiotics are substantially altered in critically ill pediatric patients.
Design/Methods:
In the context of multiple larger studies enrolling critically ill patients receiving beta-lactam antibiotics, we have enrolled a smaller subset of critically ill pediatric patients receiving cefepime, including those receiving ECMO or CRRT. Plasma samples were collected at opportune timepoints enriched for specific PK/PD metrics. Plasma concentrations of cefepime were measured by LC-MS/MS. Weight, dose, interval, and administration time were extracted from the electronic medical record. Cefepime elimination rate, half-life, peak concentration, trough, and volume of distribution were calculated for this preliminary analysis.
Results:
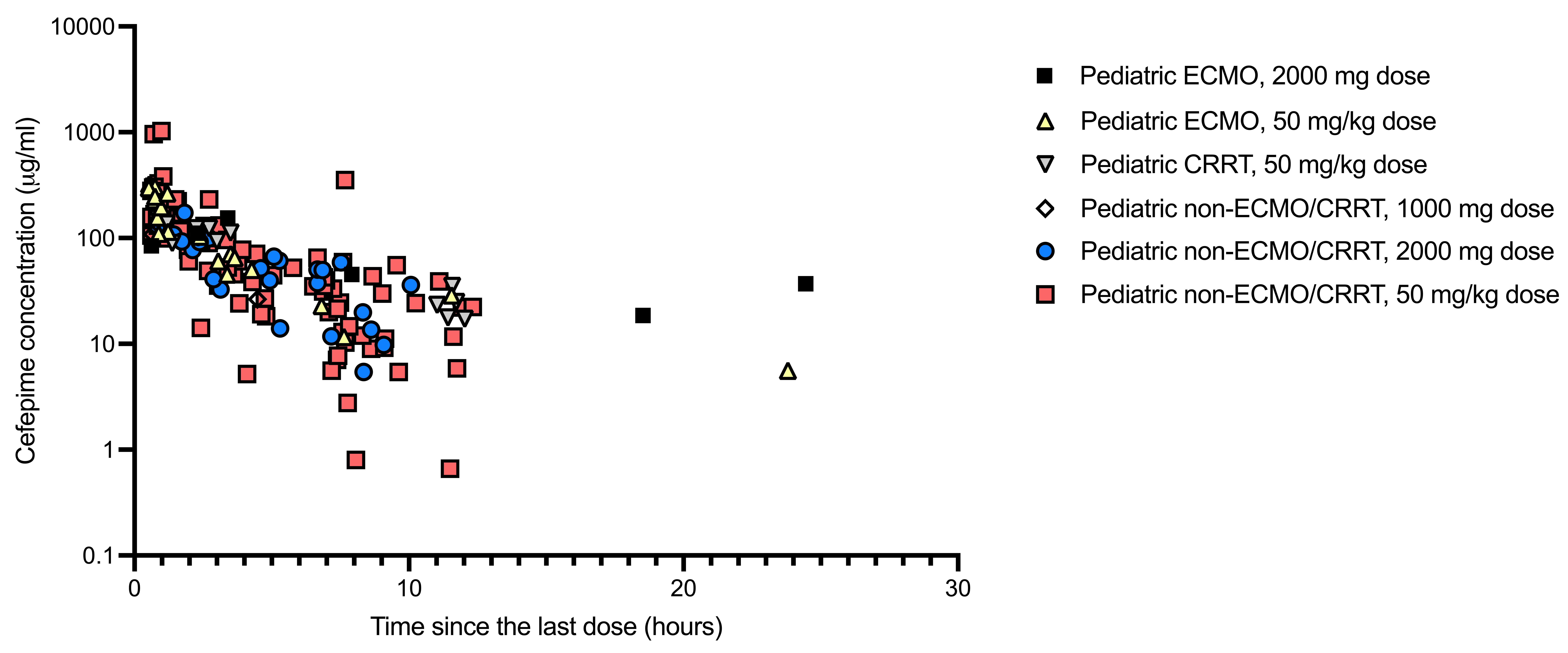
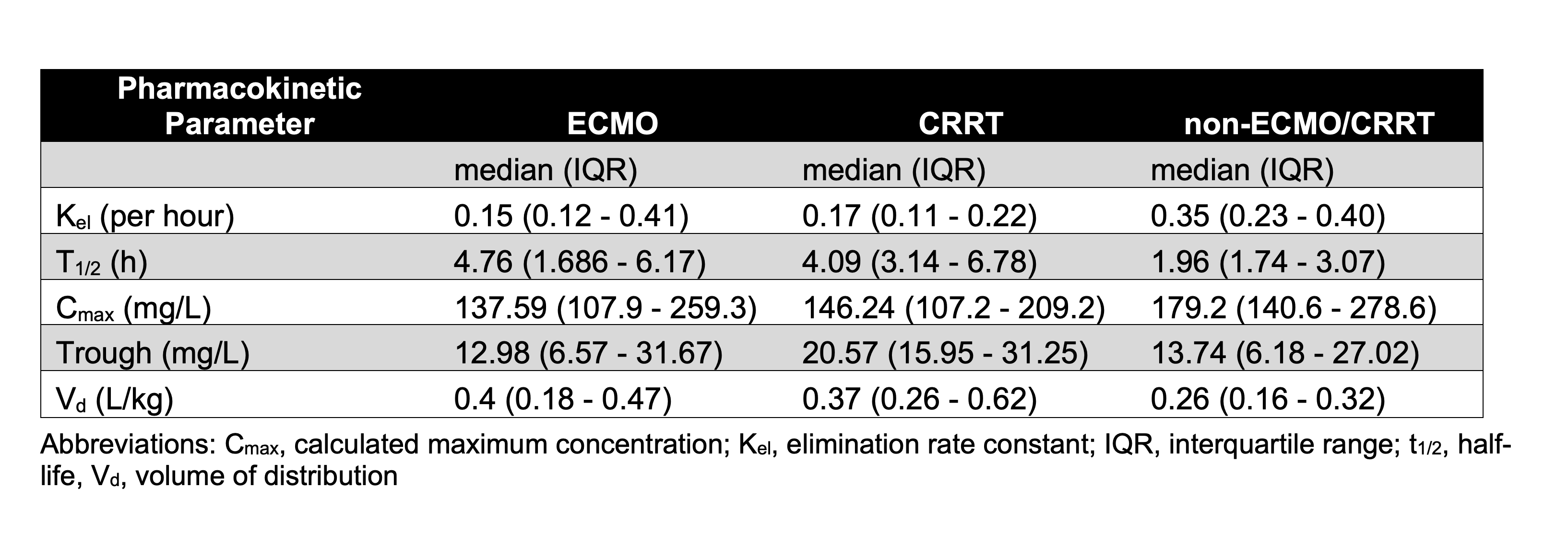
Preliminary data from 46 pediatric subjects receiving cefepime were analyzed. Of these, 4 received CRRT and 6 received ECMO. An average of 3 samples were collected per participant. The majority of patients received 50 mg/kg cefepime (n=35); one patient received 1000 mg and 10 patients received 2000 mg. The duration of infusion was 30 minutes for all doses (n=157). Figure 1 shows a plot of observed cefepime concentrations over time, by dosing regimen and group (ECMO, CRRT, or non-ECMO/CRRT). Calculated PK parameters are shown in Table 1. Notably, cefepime half-life was longer and volume of distribution was larger in both ECMO and CRRT groups compared to the non-ECMO/CRRT group.
Conclusion(s):
This study will be among the first and largest to characterize cefepime PK in the critically ill pediatric population. Variability was observed in cefepime PK parameters, particularly in the ECMO and CRRT groups compared to the non-ECMO/CRRT group. Based on these data, development of robust PK/PD models of cefepime dosing in these populations, which we are currently developing, may improve our ability to reach therapeutic targets.