Neonatal Respiratory Assessment/Support/Ventilation
Neonatal Respiratory Assessment/Support/Ventilation 5: Surfactant and NIV 2
672 - Less Invasive Surfactant Administration reduces the Rate of Invasive Mechanical Ventilation in Preterm Neonates
Publication Number: 672.443

Dionne Donald, M.D, F.A.A.P (she/her/hers)
PGY6 NICU Fellow
Stony Brook Children's Hospital
Medford, New York, United States
Presenting Author(s)
Background:
Surfactant administration remains the standard of care for infants with respiratory distress syndrome (RDS). The Intubation-Surfactant-Extubation technique reduces exposure to invasive mechanical ventilation. However, even after surfactant administration there can be a delay in the discontinuation of invasive mechanical ventilation. Less Invasive Surfactant Administration (LISA) further reduces the neonatal exposure to barotrauma and potentially reduces the severity of chronic lung disease (CLD). Various methods by which LISA can be performed include brief tracheal catheterization, laryngeal mask airway, or nebulization/aerosolization.
Objective:
To reduce the rate of invasive mechanical ventilation in the first 72 hours of life (HOL) by 25% using LISA.
Design/Methods:
This Quality Improvement (QI) initiative was approved by SUNY IRB at Stony Brook University Hospital and performed in our level III academic Neonatal Intensive Care Unit (NICU) from July 2021–2022. The project involved literature reviews, creating methodology, simulation training on the Hobart technique with a mannequin, and implementing protocol over five PDSA cycles. (Diag-1) LISA was first introduced to our unit with this QI project.
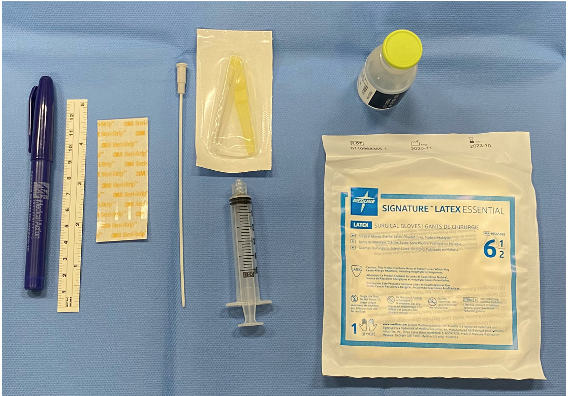
Neonates with a gestational age (GA) of 250/7 to 346/7 weeks diagnosed with RDS were included in this project. We excluded neonates < 25 weeks or >346/7 weeks GA, extramural deliveries, neonates intubated in the delivery room and with congenital anomalies. The Hobart technique was utilized for surfactant instillation into the trachea using an angiocatheter. (Pic-1) The method adapted for educational strategies included PowerPoint presentations, simulated modules for LISA administration for our medical staff, laminated bedside protocols and development of a simulation video for review.
Data were collected prospectively and reviewed monthly. The project was overseen by the NICU CLD Prevention QI Committee. Data and surfactant audits were presented at the monthly High Reliability Unit meetings.
Results:
Thirty-four patients diagnosed with RDS received surfactant. Mean GA 31 and mean birth weight 1,659grams. (Diag-2) 38% (13) received surfactant using LISA. 85% (11) of LISA patients remained on non-invasive ventilation at 72HOL. 15% (2) required intubation for a second dose of surfactant.
Conclusion(s):
LISA can be safely implemented in a level III NICU and reduce the need for invasive mechanical ventilation. We were able to decrease the number of patients who required intubation for surfactant administration within the first 72hrs of life by 32 %.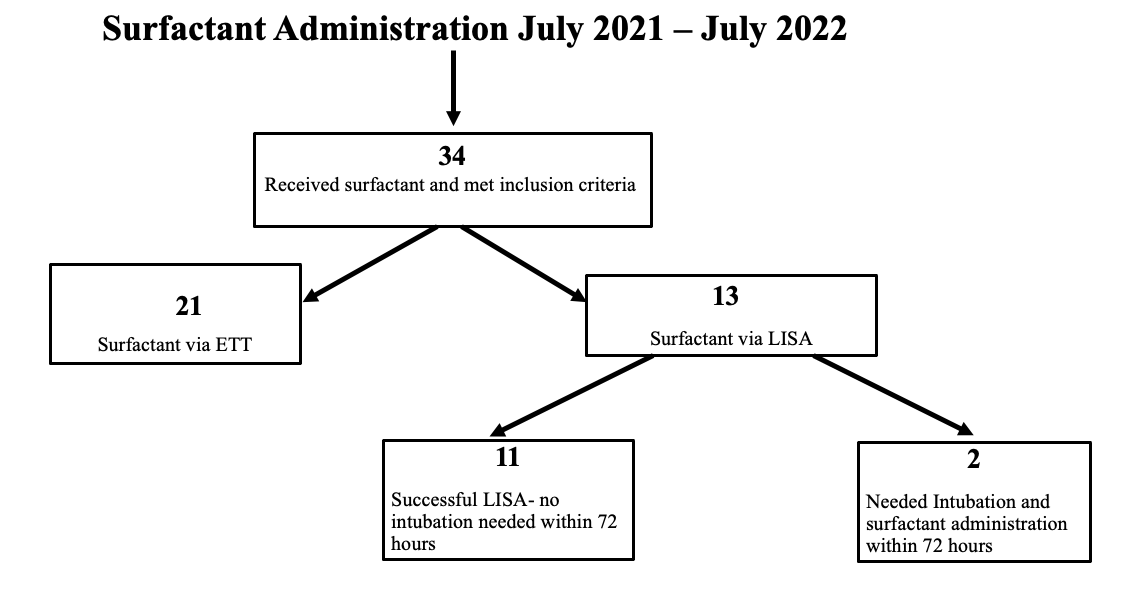
.png)
