Neonatal Infectious Diseases/Immunology
Neonatal Infectious Diseases/Immunology 5
443 - Breastmilk antibodies after primary vs. booster mRNA COVID-19 vaccination during pregnancy or postpartum
Publication Number: 443.431
- FM
Flor M. Munoz, MD (she/her/hers)
Associate Professor
Baylor College of Medicine
Houston, Texas, United States
Presenting Author(s)
Background: Breastfed infants may be protected from respiratory infections. The immune profile of breastmilk following primary vs. booster COVID-19 vaccination during pregnancy or post-partum is not well characterized.
Objective: To measure breastmilk immune profile following maternal COVID-19 mRNA primary vaccination during pregnancy and after booster vaccination during pregnancy or postpartum.
Design/Methods:
Prospective multicenter cohort study of participants enrolled after receipt of a primary 2-dose, or booster COVID-19 mRNA vaccination during pregnancy or a booster dose within 6 weeks postpartum.
Maternal blood and breastmilk samples were collected at 2 months postpartum in this substudy. IgG and IgA binding antibodies to Spike and RBD of SARS-COV-2 in breastmilk were compared in primary vs. booster vaccine recipients. The correlation between maternal Spike and RBD IgG and breastmilk Spike IgG and IgA was assessed. Maternal infection status was assessed by medical history and detection of N-protein IgG.
Results:
The study included 32 mother-infant dyads with primary 2-dose vaccination during pregnancy, 82 dyads with booster vaccination during pregnancy and 36 dyads with maternal booster within 6 weeks postpartum. The majority of infants were born at term (≥90%) and most (≥85%) were breastfeeding at 2 months postpartum.
Spike and RBD IgG and IgA were present in breastmilk in all study groups. Significantly higher IgG GMT (Spike x3 fold; RBD x3-4 fold) were measured in those who received a booster vaccination either during pregnancy or postpartum, compared with primary 2-dose vaccination during pregnancy. (Figure 1) Maternal infection increased GMT in breastmilk but numbers were low to evaluate impact. A significant positive correlation was observed between maternal serum IgG titers and breastmilk IgG and IgA at 2 months postpartum, regardless of vaccine regimen. The Spearman correlation between maternal sera Spike IgG and breastmilk antibodies was higher with breastmilk IgG (r=0.79-0.96) than with breastmilk IgA (r=0.42-0.52).(Figure 2)
Conclusion(s): These data support the potential protective effect of breastmilk antibodies in nursing infants of mothers who are vaccinated or boosted with COVID-19 mRNA vaccines during pregnancy or postpartum.
Trial Registration: clinical trials.gov; NCT05031468; https://clinicaltrials.gov/ct2/show/NCT05031468 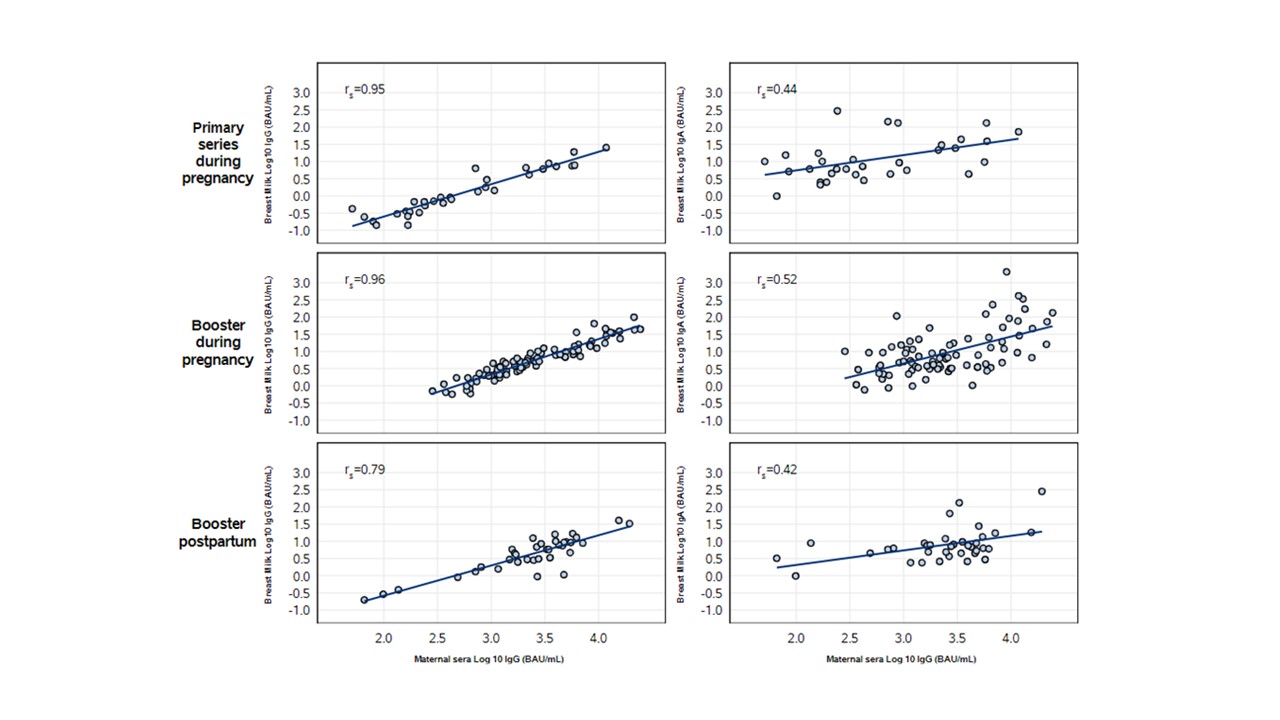
.jpg)
