Neonatal Infectious Diseases/Immunology
Neonatal Infectious Diseases/Immunology 5
453 - Primary vs. booster COVID-19 mRNA immunization during pregnancy or postpartum: Durability of immune responses in mothers and infants
Publication Number: 453.431
- FM
Flor M. Munoz, MD (she/her/hers)
Associate Professor
Baylor College of Medicine
Houston, Texas, United States
Presenting Author(s)
Background:
The durability of immunity following COVID-19 vaccination during pregnancy or postpartum and the protection afforded by a booster dose are not well characterized.
Objective: To measure immune responses in mothers and infants following maternal primary COVID-19 mRNA vaccination and booster vaccination during pregnancy or postpartum.
Design/Methods: To measure immune responses in mothers and infants following maternal primary COVID-19 mRNA vaccination and booster vaccination during pregnancy or postpartum.
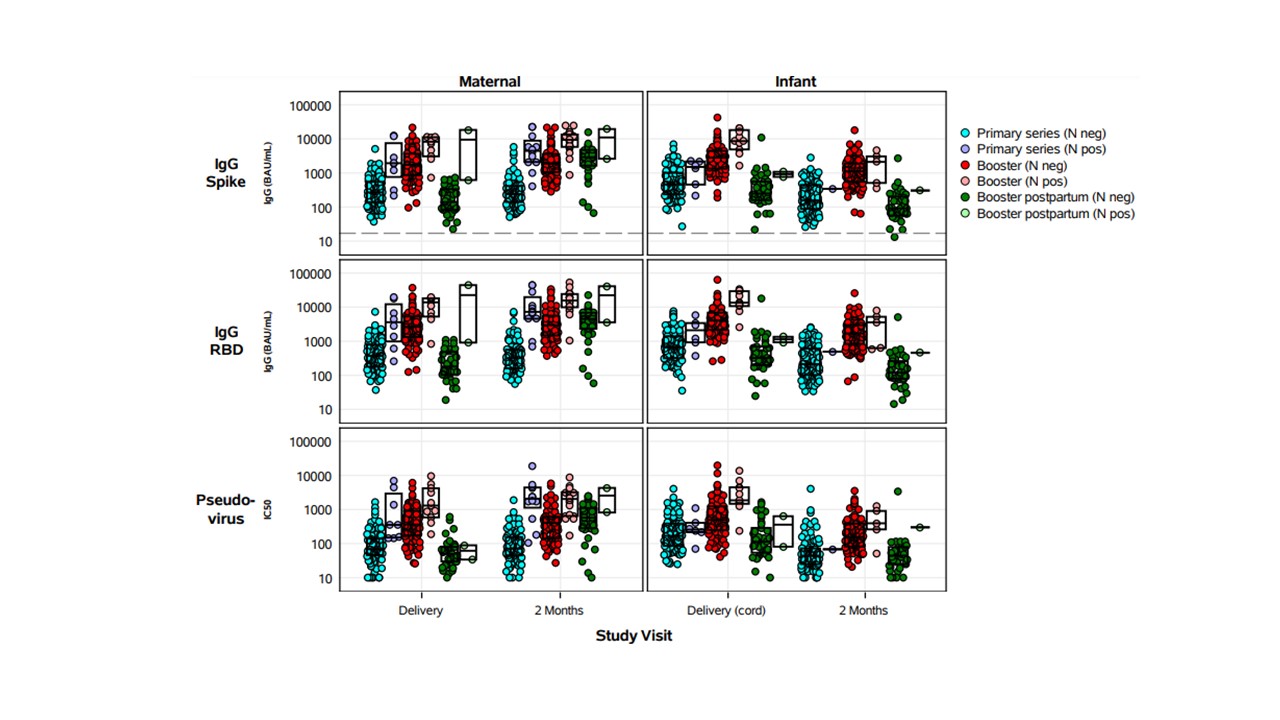
SARS-CoV-2 binding (IgG), live, and pseudovirus neutralizing antibody (nAb) titers were measured to Spike and RBD. Immune responses were compared between primary and booster vaccine recipients in maternal sera and cord blood at delivery, and maternal and infant sera at 2 months postpartum. Maternal infection status was assessed by medical history and detection of N-protein IgG.
Results: 110 maternal/infant dyads received a primary 2-dose series of either Pfizer or Moderna mRNA vaccine during pregnancy; 104 maternal/infant dyads received a booster vaccination during pregnancy, and 39 maternal/infant dyads received a postpartum booster. Maternal booster vaccination during pregnancy resulted in significantly higher GMT of Spike and RBD IgG, and pseudovirus nAb at delivery and 2 months postpartum in both mothers and infants compared with primary 2-dose series in pregnancy alone (p< 0.01 for all comparisons) (Figure 1). An increase in maternal titers was also seen at 2 months for mothers boosted postpartum. Similarly, maternal booster vaccination during pregnancy resulted in significantly higher live neutralizing antibodies to prototype, Omicron BA.1 and BA.5 strains in mothers and infants at delivery and 2 months post-delivery, compared to primary 2-dose vaccination (p< 0.01 for all comparisons). Maternal infection increased GMT but numbers are low to evaluate impact.
Conclusion(s):
Our data supports booster vaccination during pregnancy for optimal protection for the mother and newborn (through 2 months of life) against vaccine-matched circulating variants. When vaccination during pregnancy is not accomplished, post-partum vaccination can provide direct protection to the mother, and indirect protection to the infant by reducing the risk of maternal infection and disease.
Trial Registration: clinical trials.gov; NCT05031468; https://clinicaltrials.gov/ct2/show/NCT05031468