Infectious Diseases
Infectious Diseases 4
427 - The Combination of Cefazolin and LL-37 in Extended Spectrum Beta-Lactamase (ESBL) producing E. coli Urinary Tract Infections
Publication Number: 427.418

Vanessa Tamas, MD (she/her/hers)
Associate Professor of Pediatrics
Rady Children's Hospital San Diego
La Mesa, California, United States
Presenting Author(s)
Background:
Urinary tract infections (UTIs) caused by extended spectrum b-lactamase (ESBL)-producing Escherichia coli and Klebsiella pneumoniae are the most common ESBL infections in childhood. These ESBL-producing uropathogens have developed resistance to nearly all b-lactam antibiotics, including the first line UTI agent, cephalexin.
Nonetheless, children who have been diagnosed with an ESBL UTI have demonstrated both clinical and microbiologic responses to discordant antibiotics including cephalexin. This suggests that other factors such as the innate immune system, and not just antibiotic therapy may underlie the clearance of these drug-resistant uropathogens. Antimicrobial peptides, namely LL-37, protect the urinary tract from infection by two proposed mechanisms including inhibition of a bacterial biofilm formation, and at higher concentrations through direct bactericidal effect.
Objective: This study investigated the in vitro and in vivo antibacterial efficacy of cephalexin ± LL-37 against ESBL E. coli strains isolated from children diagnosed with UTIs.
Design/Methods:
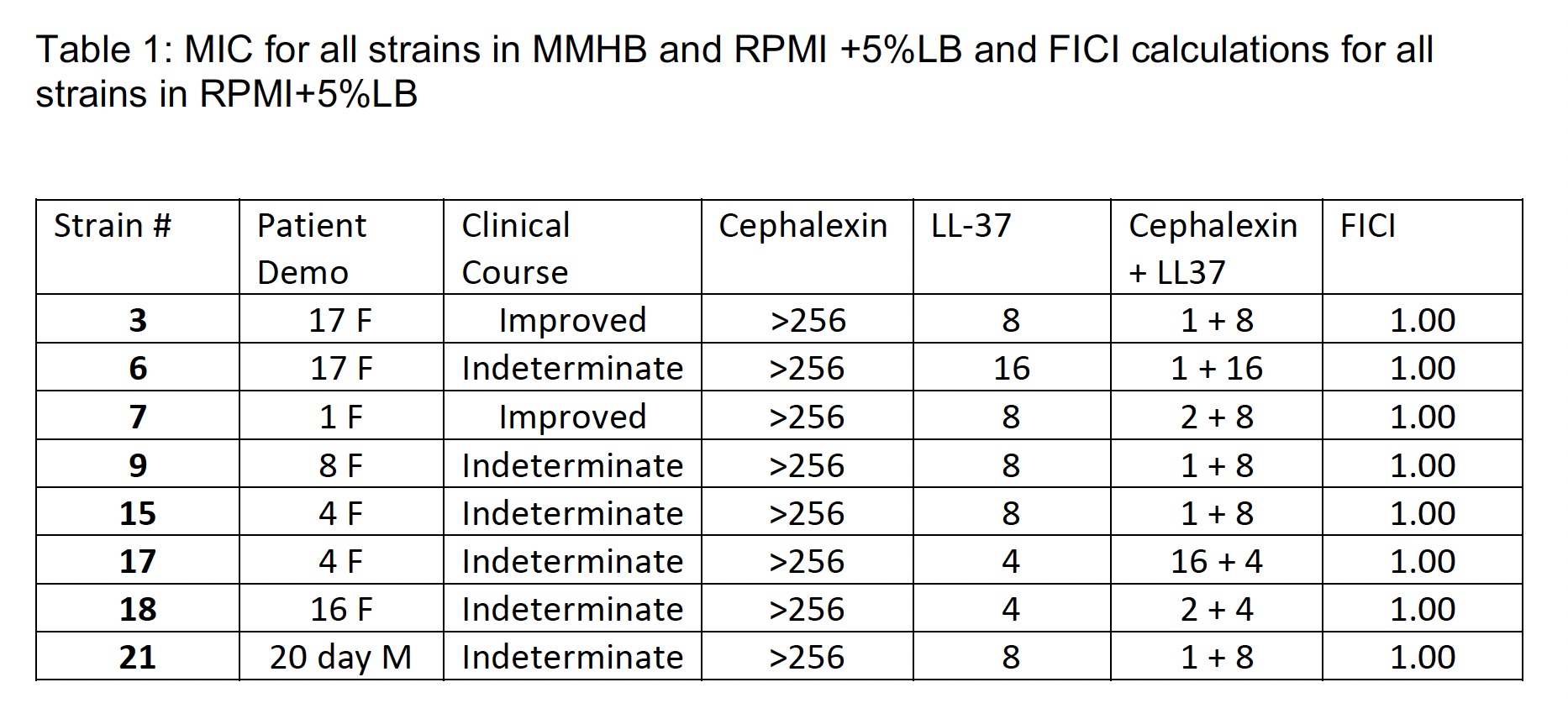
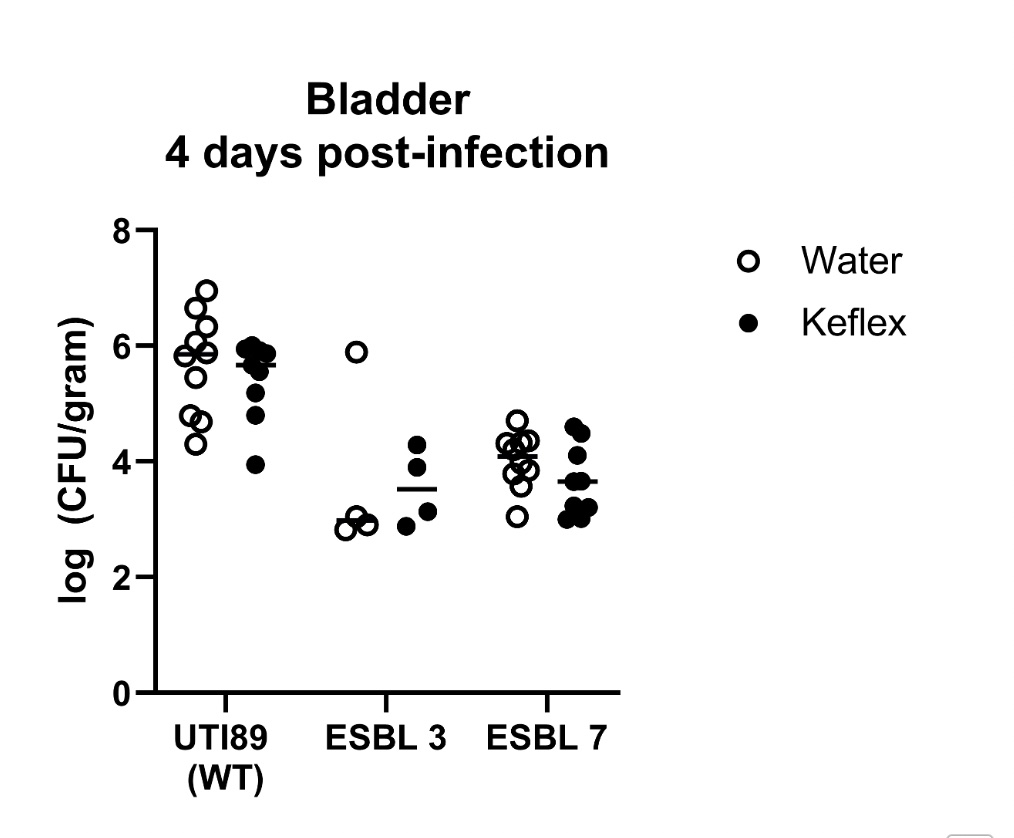
8 ESBL E. coli clinical isolates obtained from children diagnosed with UTIs were tested by minimum inhibitory concentration (MIC) in conventional Mueller-Hinton broth (MHB) in Roswell Park Memorial Institute (RPMI) physiological cell culture media supplemented with 5% Luria-Bertani (LB) broth using cephalexin. Checkerboard assays were completed with RPMI supplemented with 5% LB with cephalexin and LL-37. In vivo efficacy of cephalexin against ESBL clinical isolates was also tested in a murine bladder infection model.
Results:
The MIC of cephalexin for all strains was >256 in both media. The addition of LL-37 demonstrated indifference on checkerboard analysis. The recoverable colony forming units (CFUs) in the wild-type (WT) strain was much higher compared to the ESBL E. coli strains, irrespective of treatment condition (H2O vs cephalexin), in a murine bladder infection model. Moreover, there was no significant difference in the bacterial burden recovered from mice treated with H2O (control) versus cephalexin across the WT positive control strain or ESBL strains.
Conclusion(s):
The addition of LL-37 did not demonstrate in vitro synergy with cephalexin against the ESBL E. coli clinical isolates tested. The murine bladder infection model did not recapitulate the observed clinical efficacy of cephalexin against pediatric ESBL E. coli UTIs.