Neonatal Respiratory Assessment/Support/Ventilation
Neonatal Respiratory Assessment/Support/Ventilation 5: Surfactant and NIV 2
682 - Weaning from nasal CPAP to high flow nasal cannula is associated with increased respiratory morbidity and length of hospitalization in preterm infants born between 24-32 weeks
Publication Number: 682.443

Lisa Lavelanet, MD (she/her/hers)
Pediatric Resident
NewYork-Presbyterian Morgan Stanley Children's Hospital
New York, New York, United States
Presenting Author(s)
Background: Heated HFNC is considered to be comparable to nCPAP support in preterm infants. However, the impact of weaning from nCPAP to HFNC on respiratory outcomes and length of hospitalization (LOH) is not well known.
Objective: To determine the differences in the incidence of bronchopulmonary dysplasia (BPD; need for supplemental O2 requirement at 36 week postmenstrual age (PMA)), supplemental O2 at discharge (O2@DC), LOH, PMA at discharge, and other neonatal co-morbidities in preterm infants managed with nCPAP alone (Group-I) vs nCPAP + HFNC (Group-II).
Design/Methods: A retrospective data analysis was performed for all infants born at Morgan Stanley Children’s Hospital, Columbia University, New York at 24-32 weeks gestational age (GA) between February 2020 and June 2022. Univariate analysis was performed to compare baseline demographics, clinical characteristics, and neonatal outcomes between the two groups. A 3:1 (Group-I: Group-II) matching was then performed for significant covariates (GA, birth weight, sex, surfactant use, and incidence of PDA and NEC) and multivariable regression models were used to evaluate the risks for having respiratory morbidity or death and the LOH between the two groups.
Results:
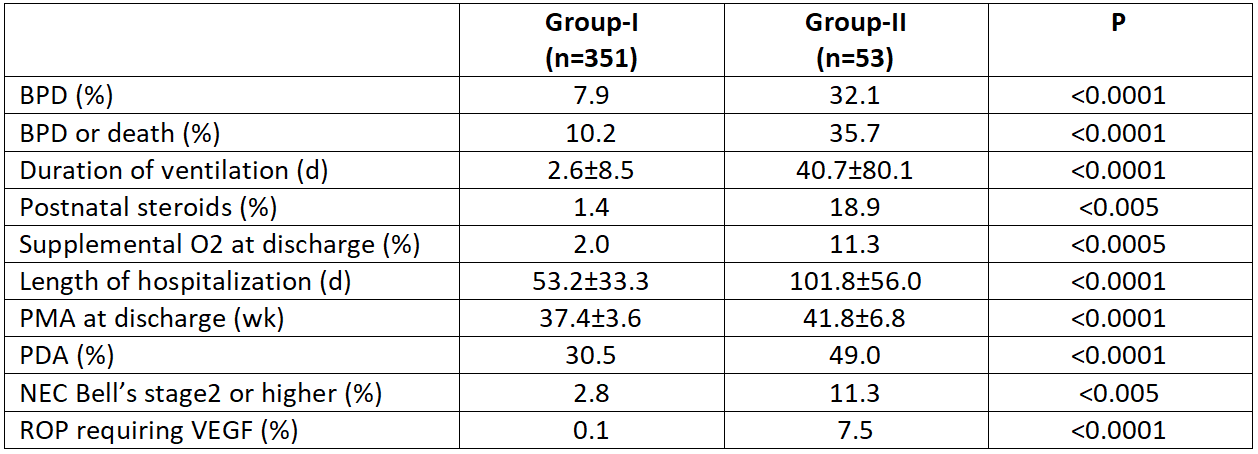
A total of 416 infants met the inclusion criteria (360 in Group-I and 56 in Group-II). On univariate analysis, infants in Group-II were born at lower GA (27.3±2.6 vs 29.8±2.2; p< 0.0001), weighed less at birth (998±412 vs 1356±440 g; p< 0.0001), and received higher rates of exogenous surfactant (35.8 vs 18.8%; p< 0.005) and caffeine therapy (49.0 vs 30.5%; p=0.01). The incidence of BPD (7.9 vs 32.1%), postnatal steroid use (1.4 vs 18.9%), O2@DC, (2.0 vs 11.3%), number of ventilator days, LOH, PMA at discharge, and several neonatal comorbidities were significantly lower in Group-I compared with Group-II (Table 1). Multivariate regression analysis revealed that HFNC use doubled the odds of BPD or death (p=0.049) and O2@DC (not significant) (Table 2), and increased the LOH by 15% (Relative risk=1.157 [1.00, 1.34], p=0.049).
Conclusion(s): In this retrospective study, weaning from nCPAP to HFNC in infants born at 28-32 weeks GA is associated with higher risk of BPD or death and prolonged hospitalization compared to nCPAP alone. A large prospective randomized controlled trial is needed to evaluate long-term safety and efficacy of HFNC in these infants.
.png)
