Neonatal Respiratory Assessment/Support/Ventilation
Neonatal Respiratory Assessment/Support/Ventilation 2: Physiology 1
331 - Non-invasive Oscillometry to Measure the Effects of Furosemide on Lung Mechanics in Extremely Preterm Infants
Sunday, April 30, 2023
3:30 PM - 6:00 PM ET
Poster Number: 331
Publication Number: 331.345
Publication Number: 331.345
Colm P. Travers, University of Alabama School of Medicine, Birmingham, AL, United States; Waldemar A. Carlo, University of Alabama School of Medicine, Birmingham, AL, United States; Kimberly Armstead, University of Alabama School of Medicine, Birmingham, AL, United States; Namasivayam Ambalavanan, University of Alabama School of Medicine, Birmingham, AL, United States

Colm P. Travers, MD
Assistant professor
University of Alabama School of Medicine
Birmingham, Alabama, United States
Presenting Author(s)
Background: Many preterm infants receive furosemide to improve lung mechanics based on data from relatively invasive pulmonary function tests. Non-invasive oscillometry measures lung resistance (R) and reactance (X), a measure of pulmonary elasticity and inertance, at the bedside without sedation.
Objective: We hypothesized that non-invasive oscillometry would reproducibly measure the effects of furosemide on lung mechanics in preterm infants.
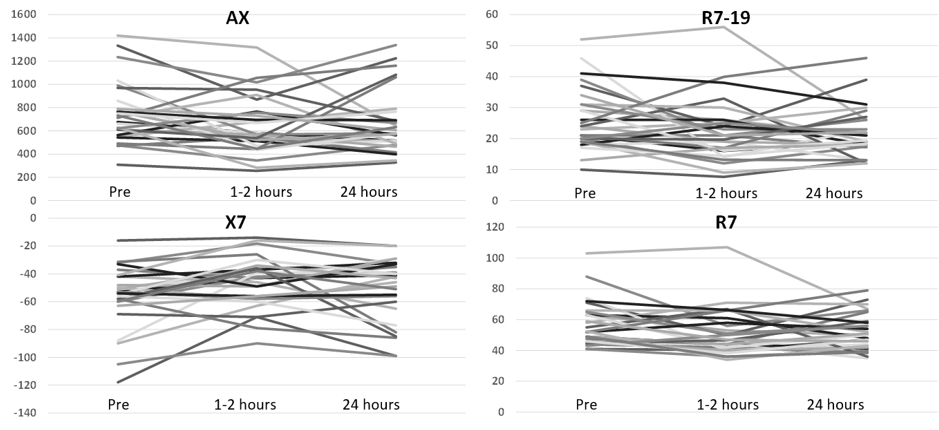
Design/Methods: Single-center prospective pre-post study including preterm infants ≥ 22w 0d off positive pressure respiratory support exposed to furosemide. Non-invasive oscillometry was performed before, 1-2 hours after, and 24 hours after clinically-indicated furosemide exposure using a Tremoflo N-100 (Thorasys, Montreal). The primary outcome was the change in the area under the reactance curve at 1-2 hours after treatment (AX). Secondary outcomes included reactance at 7 Hz (X7), resistance at 7Hz (R7), and the difference in resistance between 7Hz and 19Hz (R7-19) at 1-2 hours and 24 hours after treatment. A sample of 30 infants was required to detect a 250 cmH2O.s/L or 20% decrease (1030 cmH2O.s/L pre- vs 825 cmH2O.s/L post-treatment) in AX assuming a standard deviation of 320 cmH2O.s/L, correlation of 0.7 between pre- and post-treatment values, power of 90%, α of 5%, and approximately 10% attrition rate for inadequate/incomplete testing. Results were analyzed by paired t-tests.
Results: We tested 30 infants with a mean ± SD gestational age of 25w 6d ± 2w 1d and birth weight of 733 ± 205 grams on postnatal day 79 ± 33 from May 2021 to September 2022. Testing was complete in 28/30 (93.3%) infants and the intra-infant variability for each test session was excellent (R=0.92; R2=0.85; p< 0.001). AX decreased from 751 ± 261 cmH2O.s/L before treatment to 644 ± 246 cmH2O.s/L (mean difference, SEM, 107 ± 40; 95% CI, 24-190; p=0.01) at 1-2 hours and 660 ± 272 cmH2O.s/L (p=0.11) at 24 hours after treatment. X7 improved from -56 ± 22 cmH2O.s/L to -46 ± 18 cmH2O.s/L (p=0.01) at 1-2 hours but the difference was not significant at 24 hours (-51 ± 23 cmH2O.s/L; p=0.18). R7 decreased from 57 ± 15 cmH2O.s/L before treatment to 51 ± 15 cmH2O.s/L at 1-2 hours (p=0.02) and 52 ± 12 cmH2O.s/L (p=0.05) 24 hours after treatment. R7-19 improved from 26 ± 10 cmH2O.s/L before treatment to 22 ± 10 cmH2O.s/L (p=0.046) at 1-2 hours and 22 ± 8 cmH2O.s/L (p=0.03) at 24 hours. There were individual differences in the response to furosemide (Figure 1).
Conclusion(s): In preterm infants off positive pressure support, furosemide improved lung mechanics measured using non-invasive oscillometry.