Neonatal Respiratory Assessment/Support/Ventilation
Neonatal Respiratory Assessment/Support/Ventilation 1: Lung US - BPD
288 - The Use of Low-Dose Dexamethasone in Chronic Neonatal Respiratory Failure.
Sunday, April 30, 2023
3:30 PM - 6:00 PM ET
Poster Number: 288
Publication Number: 288.344
Publication Number: 288.344
Morcos Hanna, Texas Children's Hospital, Houston, TX, United States; Jamie Gilley, Texas Children's Hospital, Houston, TX, United States; Cynthia L. Toy, Texas Children's Hospital, Houston, TX, United States; Scott W. Osborne, Baylor College of Medicine, Houston, TX, United States

Morcos Hanna, DO (he/him/his)
Neonatology Fellow
Texas Children's Hospital
Houston, Texas, United States
Presenting Author(s)
Background: Infants born prematurely are at risk for developing bronchopulmonary dysplasia (BPD). Systemic corticosteroids are used to prevent and treat BPD by facilitating extubation in chronically ventilated very low birth weight (VLBW) infants using a low-dose dexamethasone dosing protocol (DART). Currently, there is marked variability in corticosteroid therapy used to prevent BPD. Consistent with the variability in literature, some clinicians use DART protocol outside the proposed ranges, a practice we will refer to as ad-hoc DART (Ah-DART).
Objective: To describe the outcomes of patients who received DART therapy outside of the proposed published time frame to review the importance of timing of DART therapy.
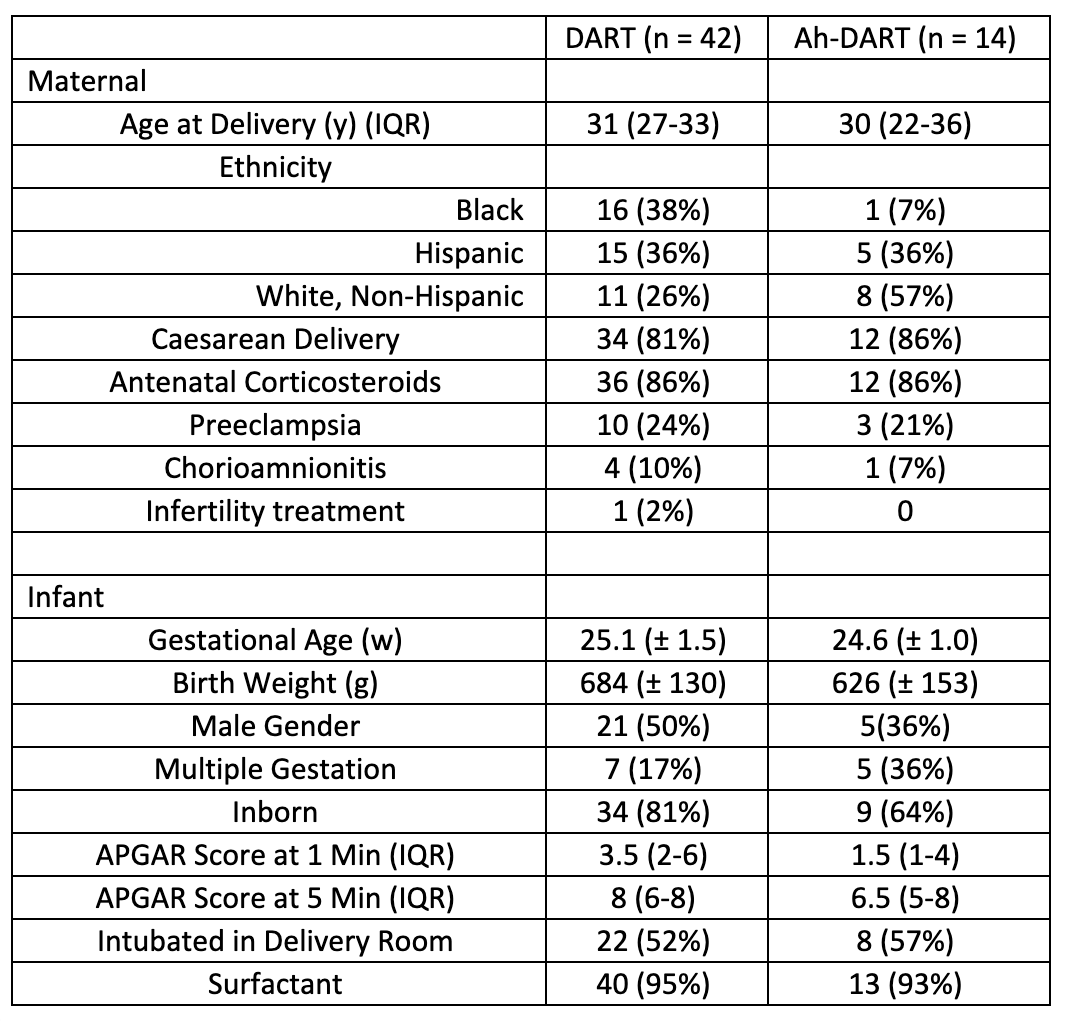
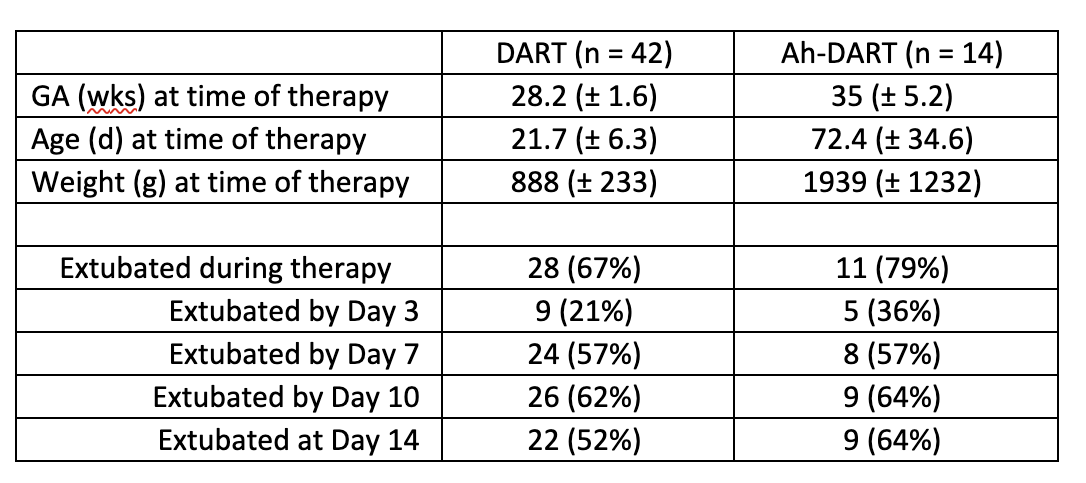
Design/Methods: A retrospective chart review of 56 patients treated with DART (n=42) and Ah-DART (n=14) at a single institution was analyzed from July 2020-June 2022. Ah-DART was defined as mechanically ventilated infants treated with the DART protocol at greater than 30 weeks gestational age and/or older than 6 weeks chronologic age. Patient demographics and variables evaluated are described in Tables 1 and 2. The NICHD definition was used to classify infants with BPD. Data were normalized and statistical significance was evaluated utilizing Fisher’s exact test.
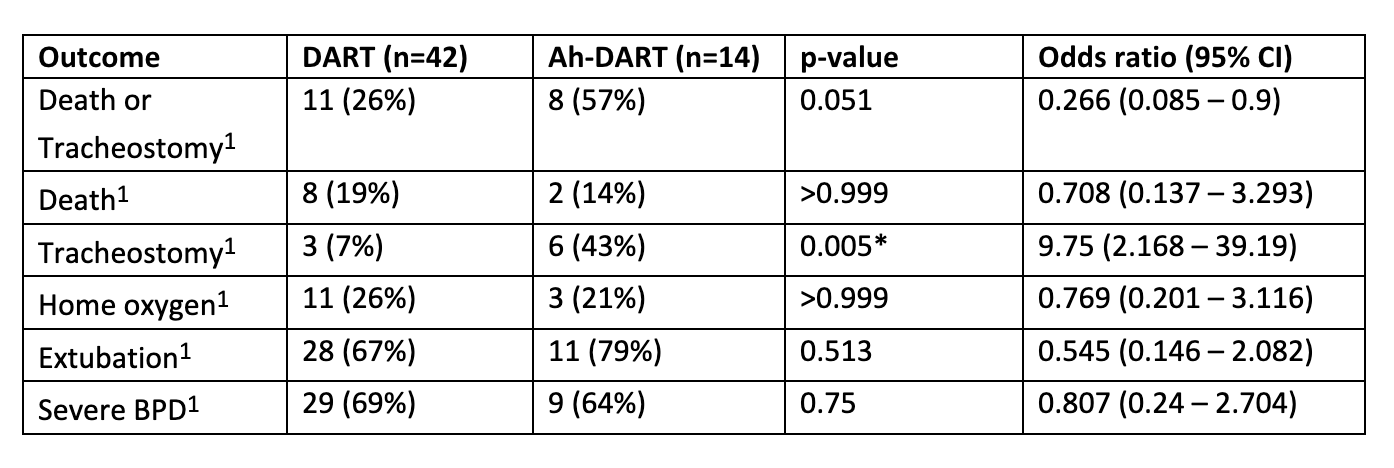
Results: Baseline demographics of the patients within the DART and Ah-DART groups were similar (Table 1). Twenty-eight (67%) infants had successful extubation with DART compared to 11 (79%) infants with Ah-DART. Only one patient receiving Ah-DART received a standard DART course prior to the Ah-DART course. Six patients required tracheostomy in the Ah-DART group compared to 3 patients in the DART group (p=0.005) (Table 3). BPD rates were similar in both groups (69% vs. 64%, p=0.75) with no difference in death.
Conclusion(s): Premature neonates treated with Ah-DART have increased need for tracheostomy when compared to infants treated with standard DART. Early DART administration may ameliorate inflammation enough to allow for extubation and more gentle non-invasive ventilation. The increased likelihood of tracheostomy in the Ah-DART group may represent long-standing inflammation that impairs ventilatory mechanics. Although the rate of extubation was similar, the Ah-DART therapy did not halt the inflammation, leading to the need for tracheostomy for safe discharge home. Further studies are needed to evaluate the benefits and long-term safety outcomes of Ah-DART in this population.