Nephrology: Transplant
Nephrology 4: Transplant
32 - Reduced Severe Neutropenia with Steroid Avoidant Immunosuppression in Pediatric Kidney Transplant Recipients
Sunday, April 30, 2023
3:30 PM - 6:00 PM ET
Poster Number: 32
Publication Number: 32.349
Publication Number: 32.349
Eliza Blanchette, Children's Hospital Colorado, Denver, CO, United States; Eric Benz, University of Colorado School of Medicine, Aurora, CO, United States; Kristen McKinnon, Children's Hospital Colorado, Aurora, CO, United States; Adrianne Sikora, Children's Hospital Colorado, Highlands Ranch, CO, United States; Margret Bock, University of Colorado School of Medicine, Denver, CO, United States
- EB
Eric Benz, MD (he/him/his)
Assistant Professor of Pediatrics
University of Colorado School of Medicine
Aurora, Colorado, United States
Presenting Author(s)
Background: Severe neutropenia with ANC < 500 (NEUT) post kidney transplant (KT) has been associated with increased recipient mortality, infection, rejection due to reductions in immunosuppression, need for hospital readmission and decreased allograft survival. Risk factors for and clinical characteristics of NEUT in pediatric KT recipients (pKTR) are poorly described.
Objective: We sought to investigate the effect of steroid avoidant immunosuppression (IS) vs. steroid based IS on timing, development and evolution of NEUT in pKTR.
Design/Methods: We completed a retrospective cohort study of pediatric kidney transplant recipients (pKTR) between 1/2016-7/2022 at our institution. Data collected included baseline demographics, relevant laboratory and medical management data, and transplant outcomes. Steroid avoidant IS was defined as discontinuation of steroid therapy after completion of 3 daily doses of induction methylprednisolone. Descriptive statistics were utilized as well as two-sample t-tests and logistic regression for tests of significance.
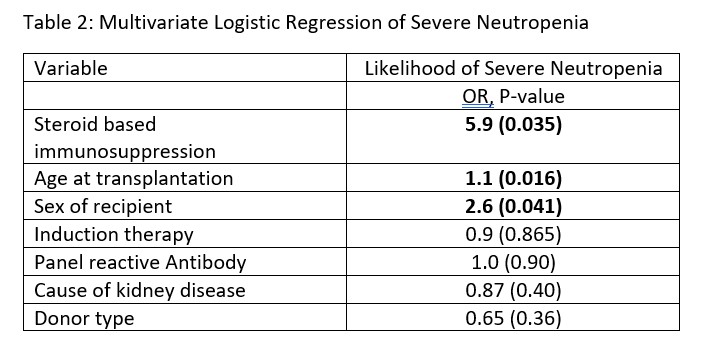
Results: 120 pKTR were included in our cohort; mean age 12.6 years (range 2-21) at time of KT and 28 (23%) with steroid avoidant IS (Table 1). Overall, 34 (29%) developed NEUT with only 7.7% of those with steroid avoidant IS vs. 34.8% in steroid based IS (p-value 0.007). On univariate logistic regression those with steroid based IS had an Odds Ratio (OR) of 6.4 for development of NEUT (p-value 0.003) (Table 2). The relationship did not change on multi-variate logistic regression with OR of 5.9 (p-value 0.005). Time to NEUT was mean of 3.7 months (range 5-365 days). Interventions for NEUT were reduction in antiproliferative (20, 61%), administration of GCSF (14, 42%), early discontinuation of valganciclovir (12, 36%), reduction in Bactrim (8, 24%) and reduction of tacrolimus (3, 9%). There was no significant increase in rejection at years 1, 2, and 3 post KT or with development of donor specific antibodies.
Conclusion(s): Increased incidence of NEUT has not been previously described as associated with steroid based IS. The mean time to NEUT occurs when steroids are typically being weaned. We hypothesize that weaning of steroids in setting of other marrow suppressant medications lead to severe suppression causing NEUT placing pKTR at risk for increased morbidity and mortality. Further investigation in larger studies is warranted.
.jpg)
