Hematology/Oncology
Hematology/Oncology 1
151 - Intravenous iron infusions in pediatric patients: a single institution retrospective review of efficacy and safety of IV iron infusions
Publication Number: 151.22

Emmalee M. Kugler, BS (she/her/hers)
Medical Student
Cooper Medical School of Rowan University
Camden, New Jersey, United States
Caitlin Strachan, BA
Medical Student
Cooper Medical School of Rowan University
Collingswood, New Jersey, United States
Presenting Author(s)
Co-Author(s)
Background:
Iron deficiency is the most common nutritional deficiency and the leading cause of iron deficiency anemia (IDA) in children worldwide. IDA is treatable with iron supplementation, commonly oral therapy. However, adherence is poor due to gastrointestinal side effects. In adult patients, IV iron is frequently used for patients unable to tolerate oral iron side effects. IV iron therapy is less commonly utilized in pediatric patients due to limited safety and efficacy data.
Objective:
This single-institution retrospective chart review of pediatric patients analyzed the safety, efficacy, and compliance of IV iron infusions compared to oral iron therapy.
Design/Methods:
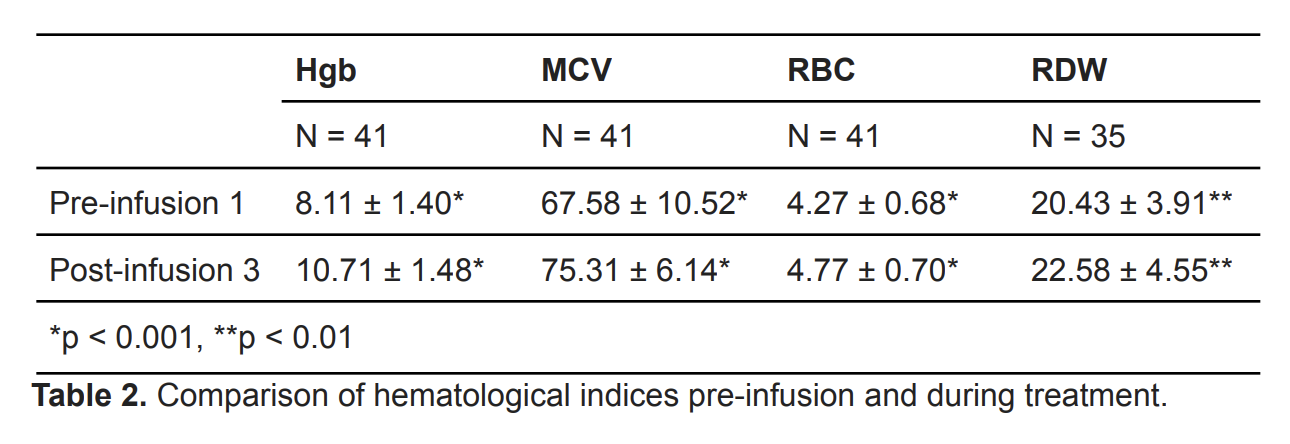
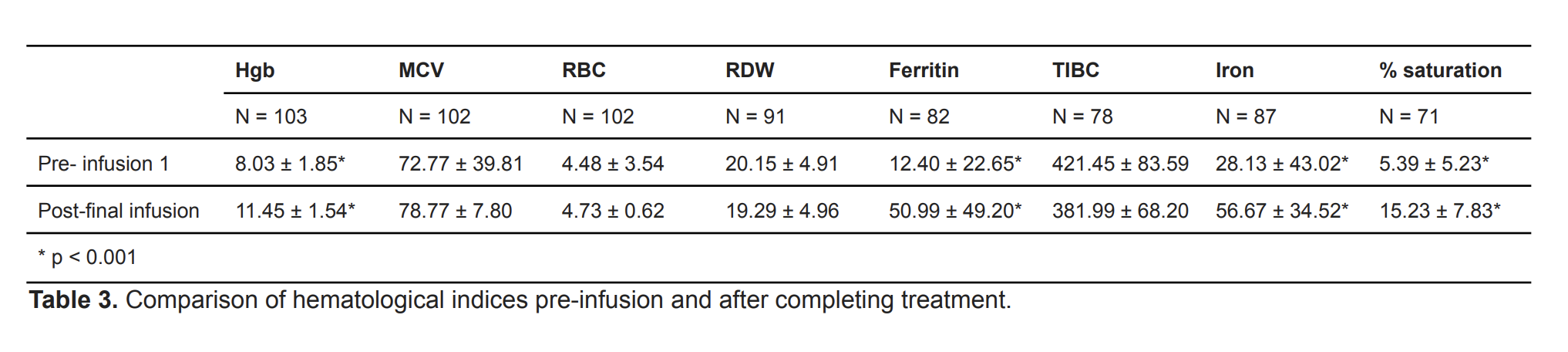
We reviewed medical records of patients aged 1-21 with various etiologies of IDA who received at least one IV iron infusion at Cooper University Hospital between 2016 and 2021 (Table 1). A paired t-test compared lab values for hemoglobin (Hgb), mean corpuscular volume (MCV), red blood cell (RBC), red cell distribution width (RDW), ferritin, total iron binding capacity (TIBC), iron stores, and iron saturation (% saturation). We analyzed adherence and adverse effects to both oral iron and IV infusions.
Results:
Our study had 107 subjects with an average age of 12.7 years (± 4.91) of which, 81.3% were female and 18.7% were male (Table 1). Hgb, MCV, and RBC were compared between pre-infusion 1 and post-infusion 3, and showed significant improvement (p < 0.001, Table 2). When comparing among age groups, improvement persisted. Hgb and iron parameters (ferritin, iron, and iron saturation) between pre-infusion 1 and post-final infusion were also significantly improved (p < 0.001, Table 3). White race and IDA due to menorrhagia showed significant improvement. Of the 107 patients, at least 70.1% were adherent to IV iron infusions and three experienced adverse reactions including rash, IV infiltration, and thrombophlebitis. Among the 86 patients initially prescribed oral iron therapy, 43.5% were adherent but 77.9% had adverse effects, of which 91% were GI-related.
Conclusion(s):
Our study demonstrated that IV iron infusions administered to pediatric patients with IDA were safe and effective. Several hematologic parameters for IDA significantly improved, including adherence, when compared to oral iron. As a single institution study, the results are limited to a regional patient population. Another limitation is that laboratory values were not collected at the third infusion for every patient due to difficult IV access. Future studies could compare patient adherence with multiple doses of IV infusions versus other single-dosing IV iron formulations..png)