Neonatal Quality Improvement
Neonatal Quality Improvement 1
650 - Use of Dextrose Gel in High-Risk Newborns in the Newborn Nursery: A Quality Improvement (QI) Initiative
Friday, April 28, 2023
5:15 PM - 7:15 PM ET
Poster Number: 650
Publication Number: 650.138
Publication Number: 650.138
Melanie Gao, NYP-Weill Cornell, New York, NY, United States; Jillian Schon, NewYork-Presbyterian Komansky Children’s Hospital, New York, NY, United States; Snezana Nena Osorio, Weill Cornell Medical College, New York, NY, United States; Erin Kelly, NewYork-Presbyterian Komansky Children’s Hospital, Mount Sinai, NY, United States; Abieyuwa Iyare, NewYork-Presbyterian Komansky Children’s Hospital, New York, NY, United States; Erika Abramson, NewYork-Presbyterian Komansky Children’s Hospital, Rye Brook, NY, United States; Rae Jean Hemway, NewYork-Presbyterian Komansky Children’s Hospital, New York, NY, United States; Priyanka Tiwari, Weill Cornell Medicine, New Hyde Park, NY, United States
- MG
Melanie Gao, MD (she/her/hers)
Pediatric Resident
NYP-Weill Cornell
New York, New York, United States
Presenting Author(s)
Background: Neonatal hypoglycemia (NH) in high risk (HR) neonates, which includes infants of diabetic mothers (IDM), large and small for gestational age (LGA, SGA) and late preterm infants (LPT), has been shown to lead to severe short-term (poor feeding, lethargy and apnea) and long-term (neurodevelopmental) consequences. Recent studies demonstrate that 40% dextrose gel (DG) is a non-invasive and inexpensive treatment that may reverse NH in HR neonates in the first 48 hours of life, with studies using up to 4-6 doses in 24 hours. DG may reduce the separation of the mother-baby dyad and decrease need for IV glucose. DG was implemented in our hospital Sept 2021.
Objective: To increase utilization of DG for HR neonates with hypoglycemia to 90% by June 2023 and reduce admission for IVF in HR neonates by 10% by June 2023.
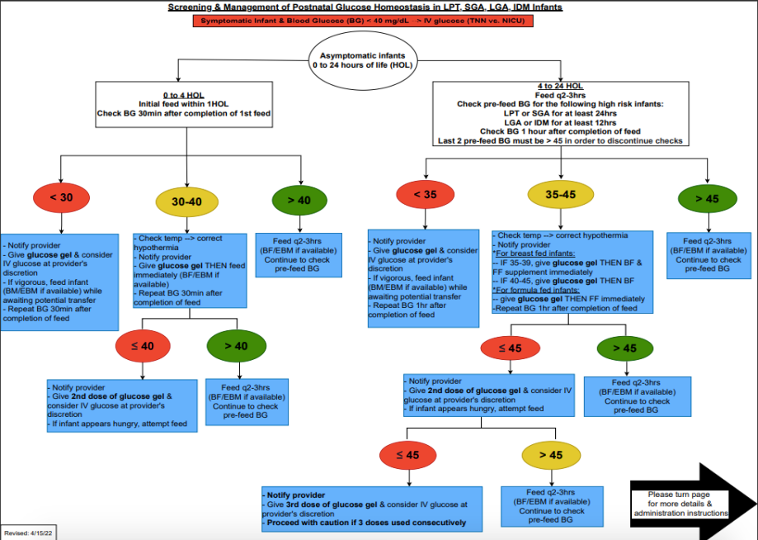
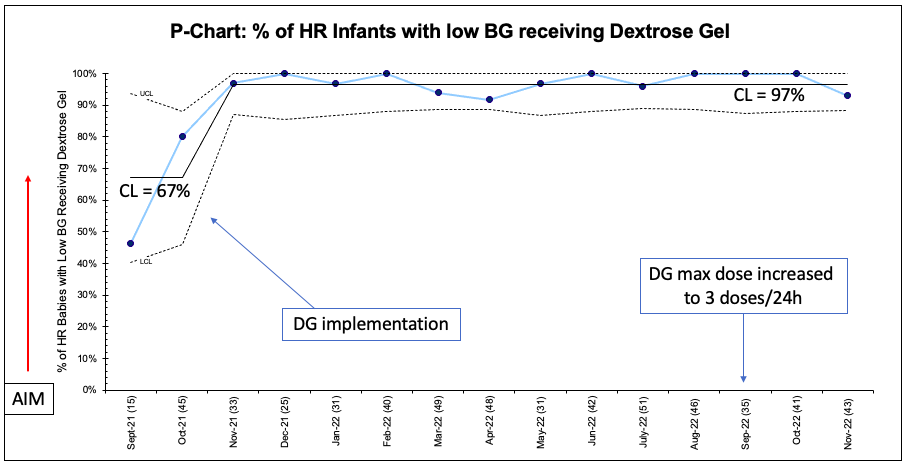
Design/Methods: This ongoing QI study utilized the Model for Improvement with a series of sequential interventions. A clinical process map was created to include indications for DG use in HR neonates (Fig 1). Baseline data was collected from Jan-Sept 2021. DG implementation was observed from Sept 2021-Dec 2022, with an increase in max dose to 3 doses / 24 hours in Sept 2022. The following family of measures were used: percentage of HR infants receiving DG (process), number of HR infants requiring IVF (outcome), LOS (balancing) and exclusive breastfeeding (BF) (balancing). Statistical process control charts (P-chart, X-bar/S-charts) were used to display and analyze data. API rules were applied to detect signal of change and special cause variation.
Results: A total of 2101 infants were included in this study: 23% SGA, 15% LGA, 18% LPT, 57% IDM, with 13% of neonates having more than one risk factor. The percentage of hypoglycemic HR infants receiving DG increased from 67% to 97% (Fig 2). 3% of all HR infants required IVF (Fig 3). 78% of all transitional nursery (TN) admissions for hypoglycemia received IVF. An unexplained transient increase in LOS from 2.1 to 3 days was initially noted, but returned to baseline following Dec 2021. 47% of HR infants were BF at discharge, unchanged from baseline. There were no adverse effects of DG usage.
Conclusion(s): We were able to implement DG successfully and safety in our nursery, thought to be secondary to the ease of the clinical process map and effective education. Our results do not demonstrate a reduction in IVF rates, which may be secondary to limiting the DG dosage to 3 doses / 24 hours. The use of DG did not alter BF rates in our study population. Next steps will focus on evaluating DG dose-dependent changes on TN admissions, IVF rates, LOS, and BF rates.
.png)
