Neonatal Clinical Trials
Neonatal Clinical Trials 1
238 - Premature infants 28-32 weeks receiving umbilical cord milking or delayed cord clamping: A Randomized non-inferiority trial
Friday, April 28, 2023
5:15 PM - 7:15 PM ET
Poster Number: 238
Publication Number: 238.127
Publication Number: 238.127
Anup Katheria, Sharp Mary Birch Neoanatal Research Institute, University of California, San Diego School of Medicine, San Diego, CA, United States; Jeff M. Szychowski, University of Alabama at Birmingham, Birmingham, AL, United States; Kathy M. Arnell, Sharp Mary Birch Hospital for Women & Newborns, San Diego, CA, United States; Wally A. Carlo, University of Alabama School of Medicine, Birmingham, AL, United States; Akila Subramaniam, University of Alabama at Birmingham, Birmingham, AL, United States; Frank Reister, University Hospital Ulm, Germany, Ulm, Baden-Wurttemberg, Germany; Jochen Essers, University Health System Children's Health, Ulm, Baden-Wurttemberg, Germany; Farha Vora, Loma Linda University Children's Hospital, LOma Linda, CA, United States; Courtney Martin, Loma Linda University School of Medicine, Loma Linda, CA, United States; Georg Schmoelzer, University of Alberta Faculty of Medicine and Dentistry, Edmonton, AB, Canada; Brenda H. Law, University of Alberta Faculty of Medicine and Dentistry, Edmonton, AB, Canada; Eugene Dempsey, university college cork, Cork, Cork, Ireland; Keelin O'Donoghue, Cork University Maternity Hospital, University College Cork, Cork, Cork, Ireland; Joseph W. Kaempf, Providence Health System, Portland, OR, United States; Mark Tomlinson, Providence St. Vincent Medical Center, Portland, OR, United States; Kevin Fulford, Sharp Grossmont Hospital for Women & Children, Severance, CO, United States; Bergen Folsom, Sharp Grossmont Hospital, San Diego, CA, United States; Simon Karam, University of Mississippi School of Medicine, Madison, MS, United States; Rachael Morris, University of Mississippi School of Medicine, Jackson, MS, United States; Toby Yanowitz, University of Pittsburgh School of Medicine, Pittsburgh, PA, United States; Stacy Beck, University of Pittsburgh School of Medicine, Pittsburgh, PA, United States; Erin A. S.. Clark, University of Utah School of Medicine, Salt Lake City, UT, United States; Tara L. DuPont, University of Utah, salt lake city, UT, United States; Manoj Biniwale, USC Keck School of Medicine, Los Angeles, CA, United States; Rangasamy Ramanathan, Keck School of Medicine of USC, Los Angeles, CA, United States; Shazia Bhat, ChristianaCare, Newark, DE, United States; Matthew K. Hoffman, Christiana Care, Newark, DE, United States; Nitin Chouthai, Saint Louis University School of Medicine, Saint Louis, MO, United States; Fayez Bany-Mohammed, University of California, Irvine, School of Medicine, Orange, CA, United States; Janardhan Mydam, John H. Stroger, Jr. Hospital of Cook County, Wheeling, IL, United States; Vivek Narendran, Cincinnati Children's Hospital Medical Center, Cincinnati, OH, United States; Fiona Wertheimer, Keck School of Medicine of the University of Southern California, Los Angeles, CA, United States; Yvonne Gollin, Specialty Obstetrics of San Diego, Solana Beach, CA, United States; Yvonne Vaucher, UCSD, La Jolla, CA, United States; Neil Norman. Finer, UCSD, La Jolla, CA, United States; Michael Varner, University of Utah School of Medicine, Salt Lake City, UT, United States; Gary R. Cutter, UAB School of Public Health, Birmingham, AL, United States; Nicole Wilson, University of Alabama at Birmingham, Birmingham, AL, United States; Wade D. Rich, Sharp Mary Birch Hospital for Women and Newborns, San Diego, CA, United States

Anup Katheria, MD
Physician
Sharp Mary Birch Neoanatal Research Institute, University of California, San Diego School of Medicine
San Diego, California, United States
Presenting Author(s)
Background: Umbilical cord milking (UCM) might be an alternative to delayed cord clamping (DCC) in preterm infants without delaying resuscitation. This PREMOD2 trial included a small strata (23-27 weeks) and large strata (28-32 weeks) gestational age infants. Enrollment in the lower gestational age strata (23-27 week infants) was discontinued due to an increase in severe intraventricular hemorrhage (IVH) in the UCM intervention. However, infants 29-32 weeks gestational age (GA) were allowed to continue enrollment with the primary focus on long term neurodevelopment outcomes. The PREMOD2 trial has completed enrollment and long-term outcomes at two years of age are being collected.
Objective: To determine whether UCM is non-inferior to DCC in premature infants 28-32 weeks GA at delivery.
Design/Methods: Our multinational, randomized controlled, non-inferiority trial included preterm infants 28-32 weeks gestational age. This includes infants in the large strata > 28 weeks GA from the start of the trial. The composite primary outcome included severe IVH or death. All data were analyzed using intention-to-treat concept.
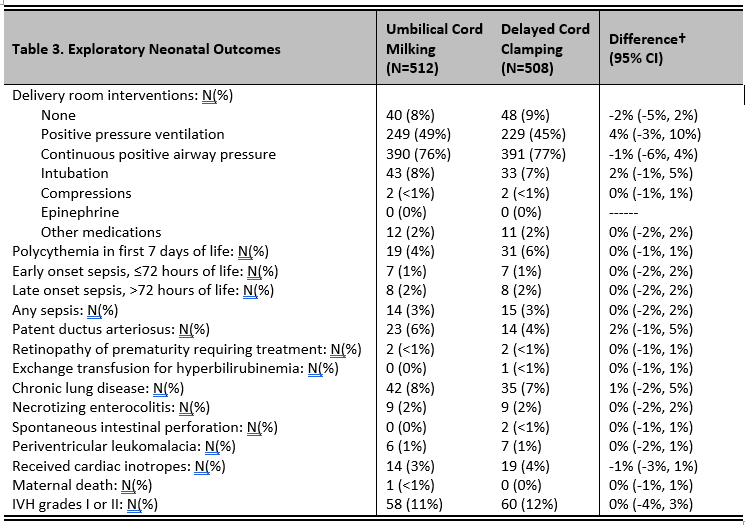
Results: A total of 1020 infants 28-32 weeks were enrolled from June 2017 until September 2022. Death or severe IVH occurred in 7/508 (1.4%) infants randomized to DCC and in 7/512 (1.4%) infants randomized to UCM (p=0.99). There were no differences in severe IVH, death, any grade IVH, hemoglobin at 4 hours of life and all exploratory outcomes (Table 3). Adherence to the randomization assignment was 95% in the UCM group and 93% in the DCC group.
Conclusion(s): In 28-32 weeks infants, there was no difference between UCM and DCC for severe IVH/death and other short-term outcomes. UCM may be a safe alternative to DCC for premature infants born at 28-32 weeks GA
.jpg)
.jpg)