Neonatal Clinical Trials
Neonatal Clinical Trials 1
236 - Facilitators and barriers to participation in a neonatal clinical trial: insights for interviews of diverse population of parents approached for neonatal research
Friday, April 28, 2023
5:15 PM - 7:15 PM ET
Poster Number: 236
Publication Number: 236.127
Publication Number: 236.127
Elliott Weiss, University of Washington, Seattle, WA, United States; Pamela K. Donohue, Johns Hopkins University School of Medicine, Baltimore, MD, United States; Susan Wootton, McGovern Medical School at the University of Texas Health Science Center at Houston, Houston, TX, United States; Emily Stephens, McGovern Medical School at the University of Texas Health Science Center at Houston, Houston, TX, United States; Stephanie L. Merhar, Cincinnati Children's Hospital Medical Center, Cincinnati, OH, United States; Mihai Puia-Dumitrescu, University of Washington School of Medicine, Seattle, WA, United States; Amanda Mercer, Portland State University, Portland, OR, United States; Ellie A. Oslin, Seattle Children's, Seattle, WA, United States; Kathryn M.. Porter, Seattle Children's Research Institute, Seattle, WA, United States; Benjamin Wilfond, University of Washington School of Medicine, seattle, WA, United States

Elliott Weiss, MD, MSME (he/him/his)
Associate Professor
University of Washington
Seattle, Washington, United States
Presenting Author(s)
Background: Low enrollment rates in neonatal clinical trials exist, especially among racial/ethnic minority populations. Past work investigating the reasons for this disparity has struggled to include the views of racial/ethnic minority parents, those with low SES, and those who declined research participation for their infant.
Objective: To describe parental reasons for and against participation in neonatal research with a focus on modifiable enrollment processes.
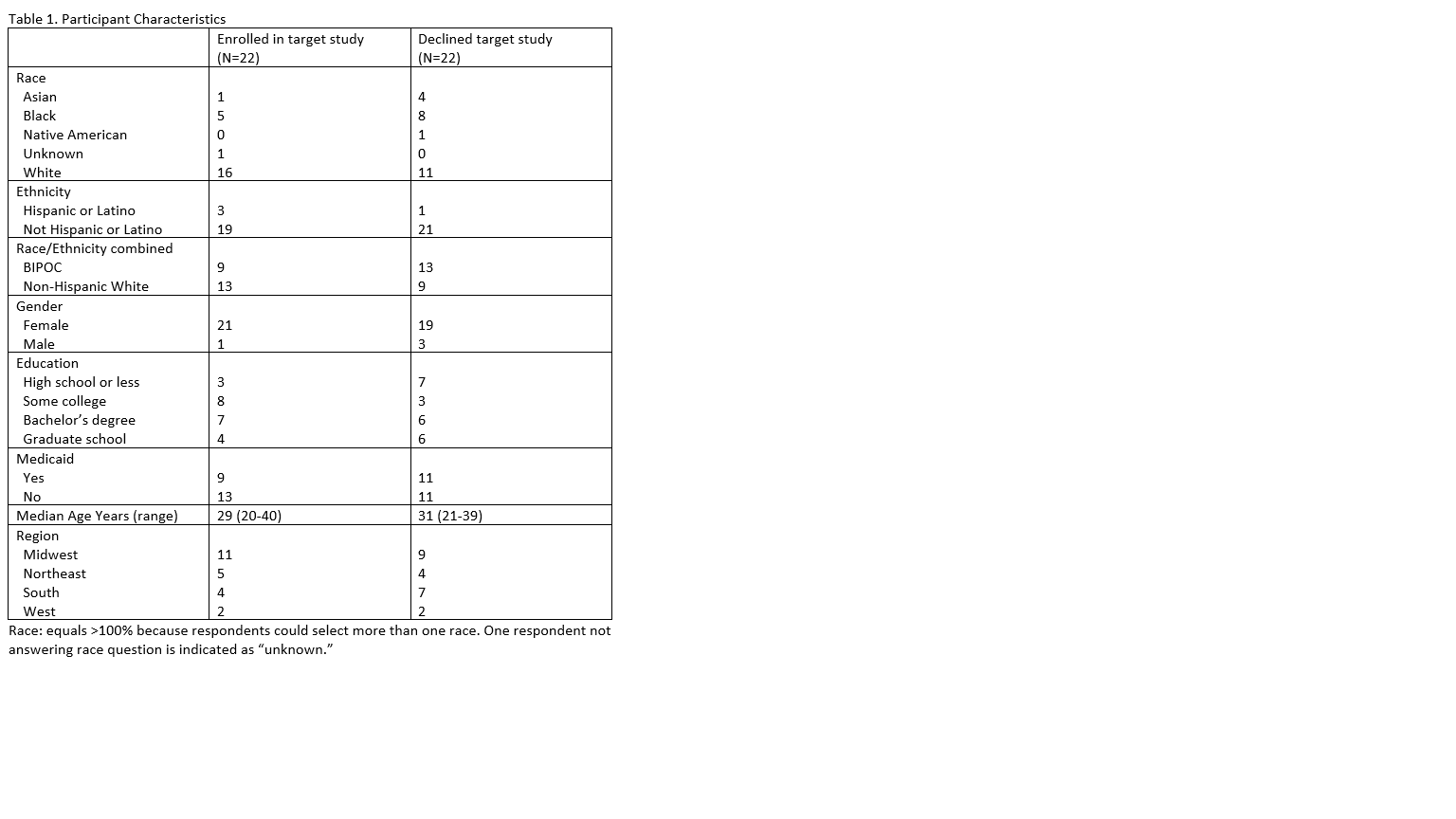
Design/Methods: We performed qualitative interviews of parents (N=44) whose infant was eligible for a neonatal clinical trial from 9 NICUs within all regions of the United States. Parents were diverse, including 22 (50%) who self-identified as racial/ethnic minorities. Half of parents interviewed declined the research study for which they were approached. Interviews were recorded and transcribed. A code book was created using standard methods and interviews were coded by two independent reviewers. Here we describe how parents reported making their decision about whether or not to participate in neonatal research.
Results: Several key themes emerged. First, the burdens of participation to the family (rather than risks and burdens to infant participant) were minimally addressed in discussions with research team members but were often deciding factors among those who declined participation. Second, altruism was a key reason for participation and an item that parents felt could be emphasized more by the research team. Third, parents often did not differentiate between baseline risks to the infant and incremental risk of study participation compared to usual care. Conceptualization of baseline risk as a risk of study participation may decrease participation, in particularly among populations with high morbidity and/or mortality. Fourth, those with greater support from others (e.g., partners, their own parents) found the enrollment decision process less stressful and were more likely to choose to participate. Fifth, privacy concerns were not a major issue for either those who declined or those who participated. Within all domains, there were minimal differences between those who identified as racial/ethnic minorities and those who identified as white non-Hispanic.
Conclusion(s): Within a diverse population of parents asked to enroll their infant in neonatal research, key reasons for and reasons against participation emerged from parent interviews. These findings can help inform clinical trialists, regulators, and funders attempting to improve recruitment processes for neonatal clinical trials.