Neonatal Clinical Trials
Neonatal Clinical Trials 1
248 - How do parents want to learn about NICU research: insights for interviews of diverse population of parents approached for neonatal research
Publication Number: 248.127

Ellie A. Oslin (she/her/hers)
Clinical Research Coordinator II
Seattle Children's
Seattle, Washington, United States
Presenting Author(s)
Background: There are known low enrollment rates in neonatal clinical trials, especially among racial/ethnic minority populations. Past work has struggled to include the views of racial/ethnic minority parents, those with low SES, and those who declined research participation.
Objective:
To describe parental preferences related to learning that their infant is eligible to participate in neonatal research to inform family-centered approaches to enrollment.
Design/Methods:
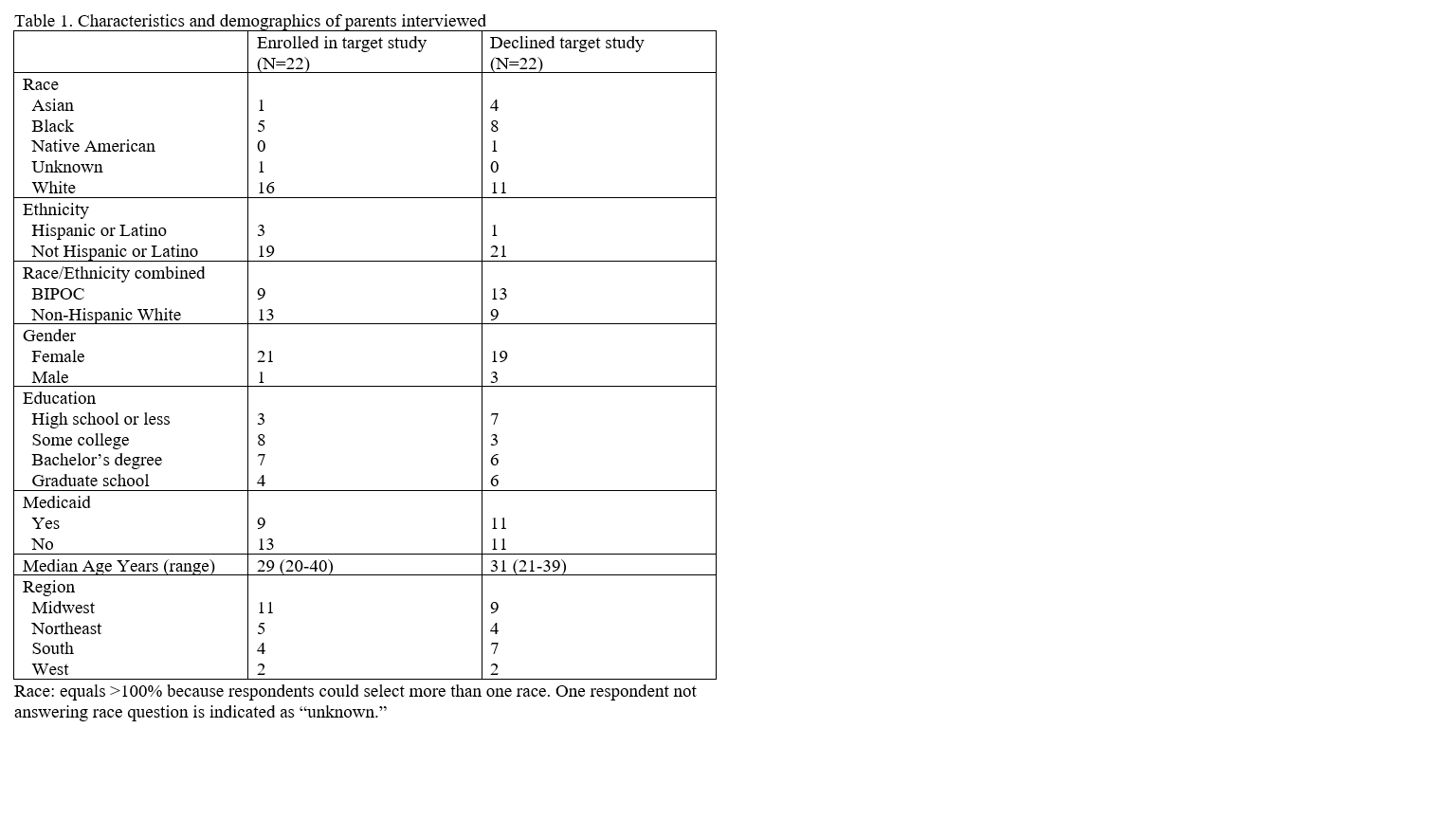
We conducted qualitative interviews of parents (N=44) at 9 clinical centers whose infant was eligible for a neonatal clinical trial. From the interviewed parents, half (N=22) declined participation in the study their infant was approached for, and half (N=22) identified as racial/ethnic minorities. Interviews were recorded and transcribed. A code book was created using standard methods and interviews were coded by two independent reviewers. Here we describe views of how parents wanted to learn about neonatal research.
Results: Key themes emerged from the data with racial/ethnic identity of parents as a factor notable across some themes. First, parental emotional state at the time of recruitment was often highly charged. Emotions, such as fear, distrust, and anxiety influenced their response to research. Second, there were differences by race/ethnicity regarding parents’ perceptions of their infant’s health, which in turn influenced their enrollment decision. Racial/ethnic minority parents were more likely to experience uncertainty surrounding the status of their infant’s health than white parents, and more commonly believed their infant was not in a condition to undergo research. Third, parents reported nuanced views related to the integration vs. distinction between research and clinical care. Most parents wanted to learn about research initially from their infant’s clinical team (e.g., nurse or doctor), but also valued content expertise of the research team later. Fourth, relationships with research staff were felt to be very important in the decision process. Parents who characterized the research staff as friendly, kind, professional, and accommodating tended to enroll their infant.
Conclusion(s):
Key themes emerged related to parental preferences for learning about NICU research. Racial/ethnic identity of parents was a meaningful factor across some themes. These findings can help inform clinical trialists, regulators, and funders attempting to improve recruitment processes for neonatal clinical trials.