Quality Improvement/Patient Safety: Improvement Science Research Methods
QI 1: Improvement Science Research Methods & Evaluation of QI Educational Interventions
681 - Clinical Usefulness as a Metric of Research Waste in Pediatric RCTs Published in 2007 and 2017
Publication Number: 681.151
.jpg)
Bartosh M. Kaminski, BSc (he/him/his)
Medical student
University of Manitoba
Winnipeg, Manitoba, Canada
Presenting Author(s)
Background:
Due to compounding errors when transitioning through the various phases of clinical research, it has been estimated that up to 85% of global research funding may be wasted on inadequately thought out and performed research. This equates to billions of dollars of wasted funding annually. Clinical usefulness as a metric of research waste has been an ongoing topic of interest since the Lancet published landmark papers in 2014.
Objective:
Using a research tool composed of 11 unique clinical usefulness criteria, the objective of our study is to examine how clinical usefulness in pediatric research has changed over two time points 10-years apart by analyzing a cohort of 600 pediatric randomized controlled trials.
Design/Methods:
We leveraged a pre-existing sample of child health randomized control trials published in 2007 and used by our team in a previous study. Using the same methods, a research librarian executed a literature search in the Cochrane Central Register of Controlled Trials for the 2017 cohort. We included the first 300 eligible citations from the randomly ordered list for each year, creating two cohorts of 300 publications for each of 2007 and 2017. Data regarding primary and secondary outcomes as well as 11 unique criteria of clinical usefulness were extracted from each RCT. Each publication was then graded using a tool created by our research team. We performed a descriptive analysis after data quality review.
Results:
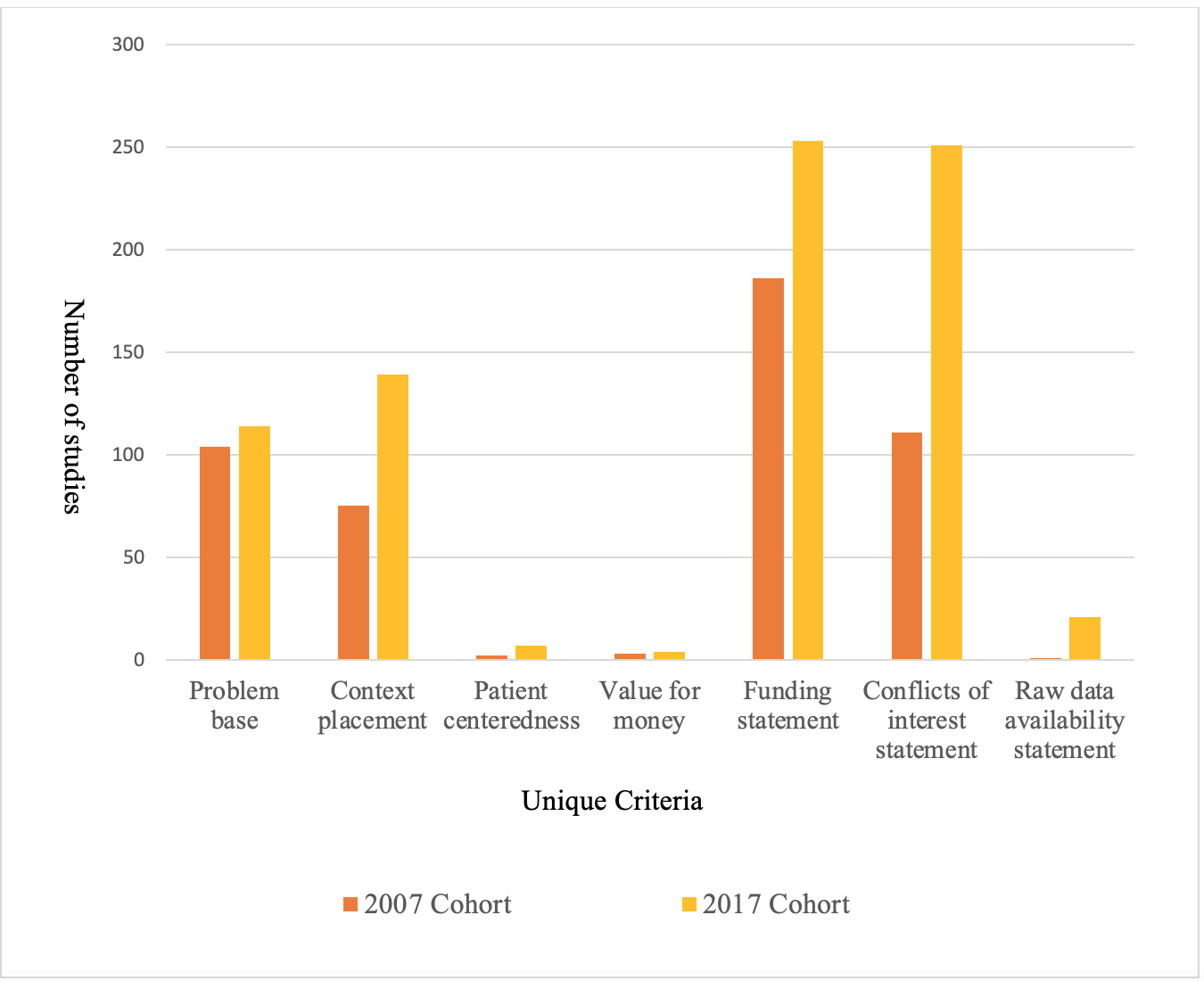
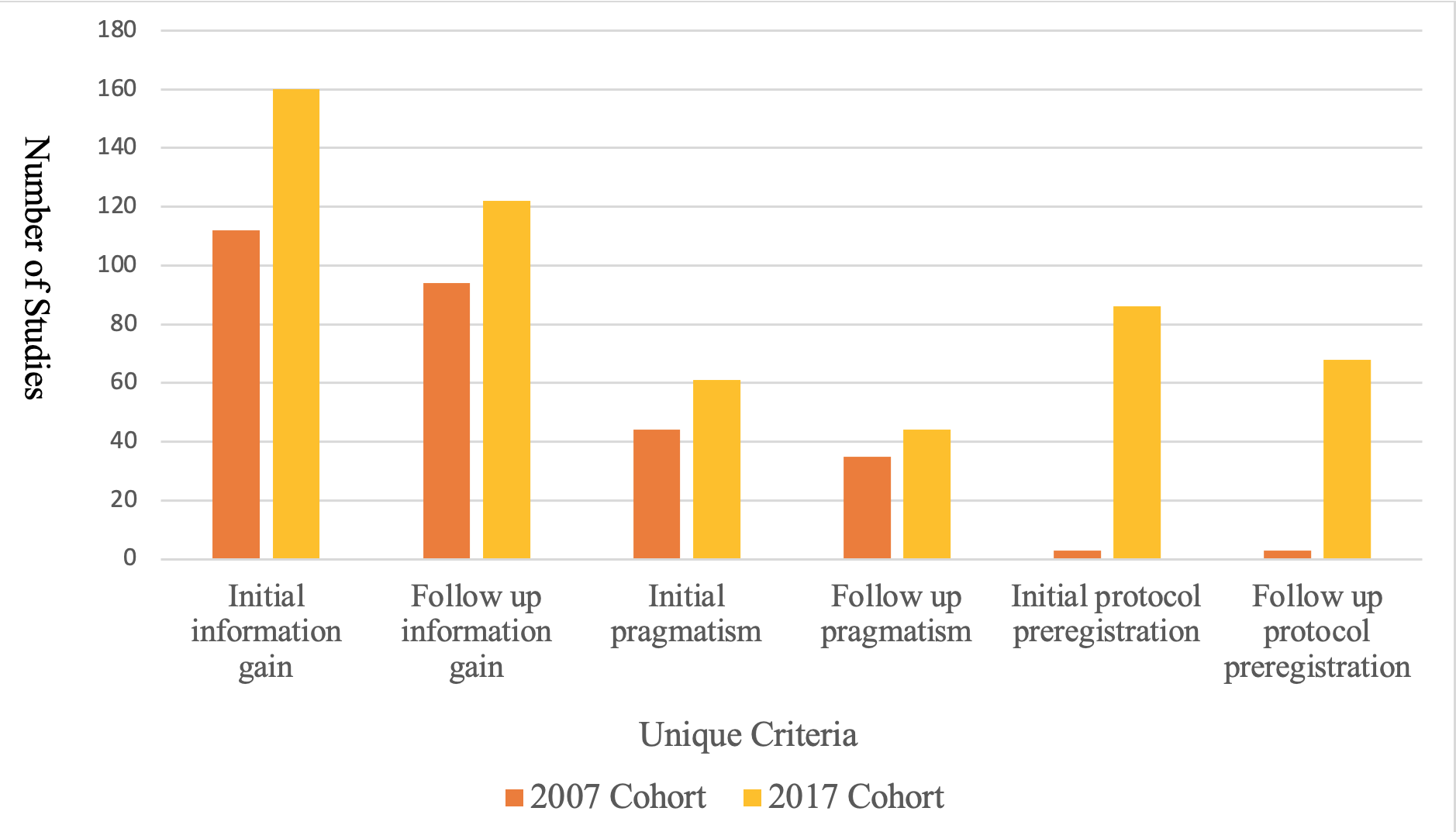
All unique criteria increased from 2007 to 2017 as shown in Figures 1 and 2. The mean score increased from 6.07 in 2007 to 9.20 in 2017 (P< 0.001). Criteria that saw the largest increase in reporting were context placement, from 75 studies in 2007 to 139 in 2017, funding source statements, from 80 studies in 2007 to 226 in 2017, and conflict of interest statements, from 81 in 2007 to 251 in 2017. Areas that need improvement are patient centeredness which had 2 studies in 2007 and 7 in 2017, value for money which had 3 studies in 2007 and 4 in 2017, and raw data availability which had 1 study in 2007 and 21 in 2017. A multivariate linear regression analysis using usefulness score as the dependent variable and multiple independent variables demonstrated that both 2017 cohort and larger sample size were significant predictors of an increased usefulness score.
Conclusion(s):
Our results demonstrate that clinical usefulness of pediatric research improved over this 10-year period. Patient centeredness and value for money are the two areas that require a great deal of improvement to maximize clinical usefulness and reduce research waste.