Neonatal Neurology: Clinical Research
Neonatal Neurology 1: Clinical 1
156 - Transcutaneous Auricular Vagus Nerve Stimulation and N-acetylcysteine may improve feeding skills in Infants of Diabetic Mothers
Publication Number: 156.135
- SG
Sandra S. Garner, PharmD (she/her/hers)
Professor
Medical University of South Carolina College of Pharmacy
Charleston, South Carolina, United States 
Dorothea Jenkins, MD (she/her/hers)
Professor of Pediatrics
Medical University of South Carolina College of Medicine
Charleston, South Carolina, United States
Presenting Author(s)
Co-Author(s)
Background:
Persistent CNS oxidative stress, present after exposure to prolonged hyperglycemia during pregnancy, has been shown to impact the ability to learn motor skills. Transcutaneous auricular vagus nerve stimulation (taVNS) paired with motor activity may improve motor rehabilitation in infants who are failing oral feeding, but was not effective in a prior study of taVNS in infants of poorly controlled diabetic mothers (8 out of 12 total IDM received a G-tube). Further, oxidative stress measured by CNS glutathione (GSH) interacted with IDM status to predict infants who attained full oral feeds (p=0.040). We hypothesized that N-acetylcysteine (NAC) would mitigate CNS oxidative stress, which would allow neuroplasticity and oromotor learning with taVNS compared with IDM treated with taVNS alone.
Objective: To determine if enteral NAC increases CNS glutathione and improves daily po feeding volumes with taVNS-paired feeding treatment in 10 IDM failing oral feeds, compared to a previous cohort of 12 IDM treated with taVNS-paired feeding alone.
Design/Methods: We enrolled 10 IDM who were determined to need G-tubes for oral feeding failure in an open label pilot trial (planned n=10). NAC 75-100mg/kg q6h was administered by n.g. for 4d prior to delivering NAC with taVNS paired with suck-swallow during 2 daily feeds for 2-3weeks. We performed MRS and diffusion MRI before NAC, before taVNS, and at the end of treatment. We measured NAC plasma concentrations around NAC dose on day3-4 with MRS. We compared daily increase in po feeding volumes between IDM treated with NAC+taVNS versus taVNS alone.
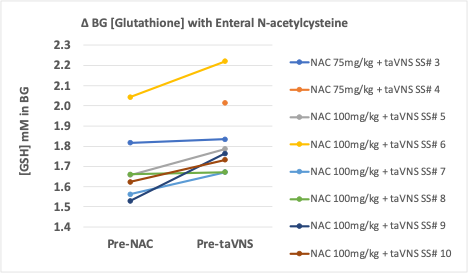
Results: Mean GA at NAC start was 41±1.1 weeks. Six of 10 IDM infants attained full oral feeds and increased daily po volumes during 14d of NAC+taVNS (86±36ml/kg/d) compared to 14d before treatment (47±29ml/kg/d; p< 0.00001). Four infants did not improve intake and required G-tube with NAC+taVNS vs 9/12 with taVNS alone. [GSH] in the BG was mean of 1.68mM at baseline, and increased by +0.13±0.08mM in the 6 IDM who received 100mg/kg/dose NAC (p=0.010) versus -0.05mM in 6 IDM who received taVNS alone. PK show that 100mg/kg/dose n.g. q 6h resulted in max serum concentration of 40 mcg/ml and AUC of 80 mcg*hr/ml.
Conclusion(s):
In IDMs failing oral feeding, 60% treated with NAC and taVNS paired with bottle feeding achieved full oral feeds, compared with 25% in our historical cohort treated with taVNS alone. Enteral NAC at 100mg/kg every 6h is adequate to significantly increase GSH concentrations in the BG. NAC plus taVNS may help avoid G-tube placement better than taVNS alone in infants with persistent CNS oxidative stress.