Back
Background: INTERGROWTH-21st growth curves generated with longitudinal data collected prospectively have more accurate measurements than traditional growth curves generated with cross-sectional data collected retrospectively.
Objective: To determine if adverse growth outcomes defined with z-scores calculated using the INTERGROWTH-21st growth curves can accurately predict the risk of cognitive delay in extremely preterm infants born at ≤26 weeks’ gestation.
Design/Methods: Extremely preterm infants of 24 to 26 weeks' gestation admitted to NICHD Neonatal Research Network neonatal intensive care units from 2008 to 2018 and assessed for cognitive outcomes at 2 years of age were included. Z-scores for weight, length, and head circumference were calculated using the INTERGROWTH-21 standards. The final sample was randomly split into development and testing samples. Classification and regression trees (CART) were used to identify the most appropriate cut-offs for weight z-score at 36 weeks postmenstrual age (PMA) and for decline in weight z-score from birth to 36 weeks PMA to predict risk of cognitive delay (defined as Bayley-III cognitive composite score < 85).
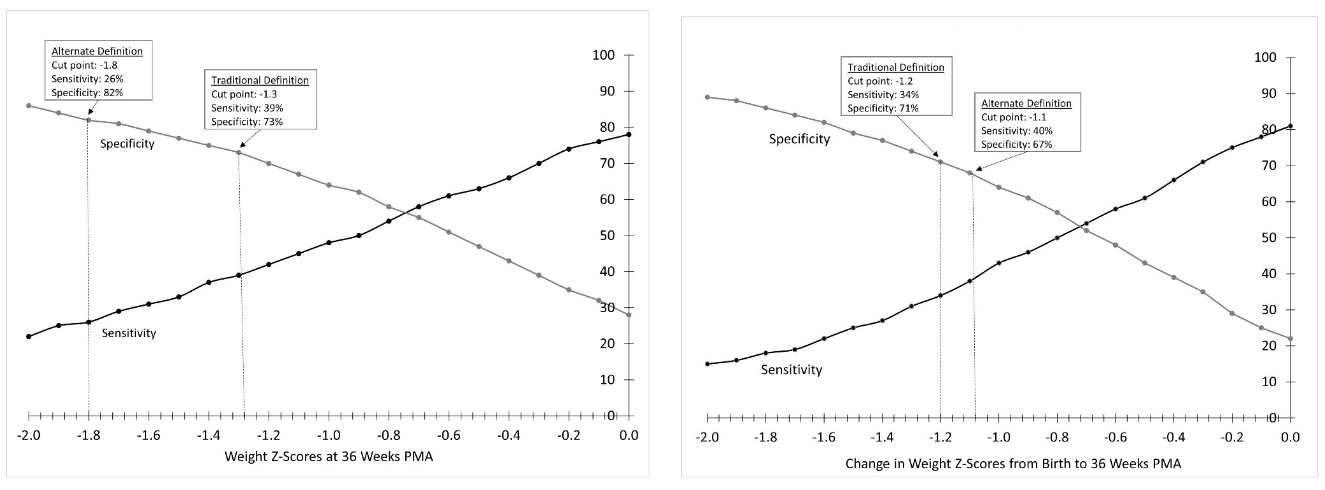
Results: A final analytic sample of 5,393 infants was randomly split into development (n=2,696) and testing (n=2,697) samples. A weight z-score at 36 weeks PMA of ≤ -1.8 and a weight z-score decline from birth to 36 weeks PMA of ≤ -1.1 were identified as the optimal cut-offs that indicate a greater likelihood of having a cognitive delay. Both cut-offs were significantly associated with a higher risk of cognitive delay in analyses adjusted for year, center, in-hospital morbidities, and maternal/neonatal demographics (aRR: 1.33 [95% CI: 1.15, 1.54] and aRR: 1.18 [95% CI: 1.03, 1.35], respectively). Both cut-offs were associated with lower length and head circumference z-scores. A weight z-score at 36 weeks PMA of ≤ -1.8 had the highest specificity for cognitive delay at 2 years of age (82%). A weight z-score decline from birth to 36 weeks PMA of ≤ 1.1 had the highest sensitivity (40%) (Figure).
Conclusion(s): As a diagnostic test derived from INTERGROWTH-21st standards, weight z-score at 36 weeks PMA has the highest specificity (82%) to predict cognitive delay. Weight z-score decline from birth to 36 weeks PMA has the highest sensitivity (40%) to predict cognitive delay, but may be too low to guide clinical practice as a stand-alone predictor. Since both growth outcomes are associated with a higher risk of cognitive delay, they could be added to predictive equations aimed at determining the probability of cognitive delay in extremely preterm infants.
Neonatal Follow-up
NICU Follow Up and Neurodevelopmental 2: Neonatal Growth, Nutrition and the Brain
214 - Postnatal Growth as Stand-Alone Predictor of Cognition at 2 Years of Age in Extremely Preterm Infants
Friday, April 28, 2023
5:15 PM – 7:15 PM ET
Poster Number: 214
Publication Number: 214.144
Publication Number: 214.144
Ariel A.. Salas, University of Alabama School of Medicine, Birmingham, AL, United States; Waldemar A. Carlo, University of Alabama School of Medicine, Birmingham, AL, United States; Carla Bann, RTI International, Apex, NC, United States; Edward F.. Bell, University of Iowa, Iowa City, IA, United States; Tarah T. Colaizy, University of Iowa Stead Family Children's Hospital, Iowa City, IA, United States; Noelle Younge, Duke University School of Medicine, Durham, NC, United States; Myriam Peralta-Carcelen, University of Alabama at Birmingham, Birmingham, AL, United States; Namasivayam Ambalavanan, University of Alabama School of Medicine, Birmingham, AL, United States; Brenda Poindexter, Children’s Healthcare of Atlanta and Emory University, Atlanta, GA, United States; NICHD Neonatal Research Network, Eunice Kennedy Shriver National Institute of Child Health and Human Development, Bethesda, MD, United States

Ariel A. Salas, MD, MSPH
Associate Professor
University of Alabama School of Medicine
Birmingham, Alabama, United States
Presenting Author(s)
Background: INTERGROWTH-21st growth curves generated with longitudinal data collected prospectively have more accurate measurements than traditional growth curves generated with cross-sectional data collected retrospectively.
Objective: To determine if adverse growth outcomes defined with z-scores calculated using the INTERGROWTH-21st growth curves can accurately predict the risk of cognitive delay in extremely preterm infants born at ≤26 weeks’ gestation.
Design/Methods: Extremely preterm infants of 24 to 26 weeks' gestation admitted to NICHD Neonatal Research Network neonatal intensive care units from 2008 to 2018 and assessed for cognitive outcomes at 2 years of age were included. Z-scores for weight, length, and head circumference were calculated using the INTERGROWTH-21 standards. The final sample was randomly split into development and testing samples. Classification and regression trees (CART) were used to identify the most appropriate cut-offs for weight z-score at 36 weeks postmenstrual age (PMA) and for decline in weight z-score from birth to 36 weeks PMA to predict risk of cognitive delay (defined as Bayley-III cognitive composite score < 85).
Results: A final analytic sample of 5,393 infants was randomly split into development (n=2,696) and testing (n=2,697) samples. A weight z-score at 36 weeks PMA of ≤ -1.8 and a weight z-score decline from birth to 36 weeks PMA of ≤ -1.1 were identified as the optimal cut-offs that indicate a greater likelihood of having a cognitive delay. Both cut-offs were significantly associated with a higher risk of cognitive delay in analyses adjusted for year, center, in-hospital morbidities, and maternal/neonatal demographics (aRR: 1.33 [95% CI: 1.15, 1.54] and aRR: 1.18 [95% CI: 1.03, 1.35], respectively). Both cut-offs were associated with lower length and head circumference z-scores. A weight z-score at 36 weeks PMA of ≤ -1.8 had the highest specificity for cognitive delay at 2 years of age (82%). A weight z-score decline from birth to 36 weeks PMA of ≤ 1.1 had the highest sensitivity (40%) (Figure).
Conclusion(s): As a diagnostic test derived from INTERGROWTH-21st standards, weight z-score at 36 weeks PMA has the highest specificity (82%) to predict cognitive delay. Weight z-score decline from birth to 36 weeks PMA has the highest sensitivity (40%) to predict cognitive delay, but may be too low to guide clinical practice as a stand-alone predictor. Since both growth outcomes are associated with a higher risk of cognitive delay, they could be added to predictive equations aimed at determining the probability of cognitive delay in extremely preterm infants.

