Neonatal Quality Improvement
Neonatal Quality Improvement 2
657 - An Interdisciplinary Quality Improvement Initiative Results in Individualized Supplementation to Support Bone Health in High-Risk Neonates
Friday, April 28, 2023
5:15 PM - 7:15 PM ET
Poster Number: 657
Publication Number: 657.139
Publication Number: 657.139
Priya V. Creed, Indiana University Riley Hospital for Children, Indianapolis, IN, United States; Katie Huff, Indiana University School of Medicine, Indianapolis, IN, United States; Beatrice Stefanescu, Riley Hospital for Children at Indiana University Health, Indianapolis, IN, United States
- PC
Priya V. Creed, MD (she/her/hers)
Fellow
Indiana University
Indianapolis, Indiana, United States
Presenting Author(s)
Background: The best approach to metabolic bone disease of prematurity (MBDP) is timely screening with the goal of optimizing nutrition early. Unfortunately, there is significant practice variation in MBDP screening practices in neonatal intensive care units (NICU). This was evident at our institution through our prior needs assessment data which showed a critical need for practice improvement.
Objective: The SMART aim for this study is to increase screening for MBDP for all infants born < 1500 grams and < 32 gestational weeks with a bundle of serum calcium, phosphorous, and alkaline phosphatase at four to six weeks of life from a baseline of 29% to 90%.
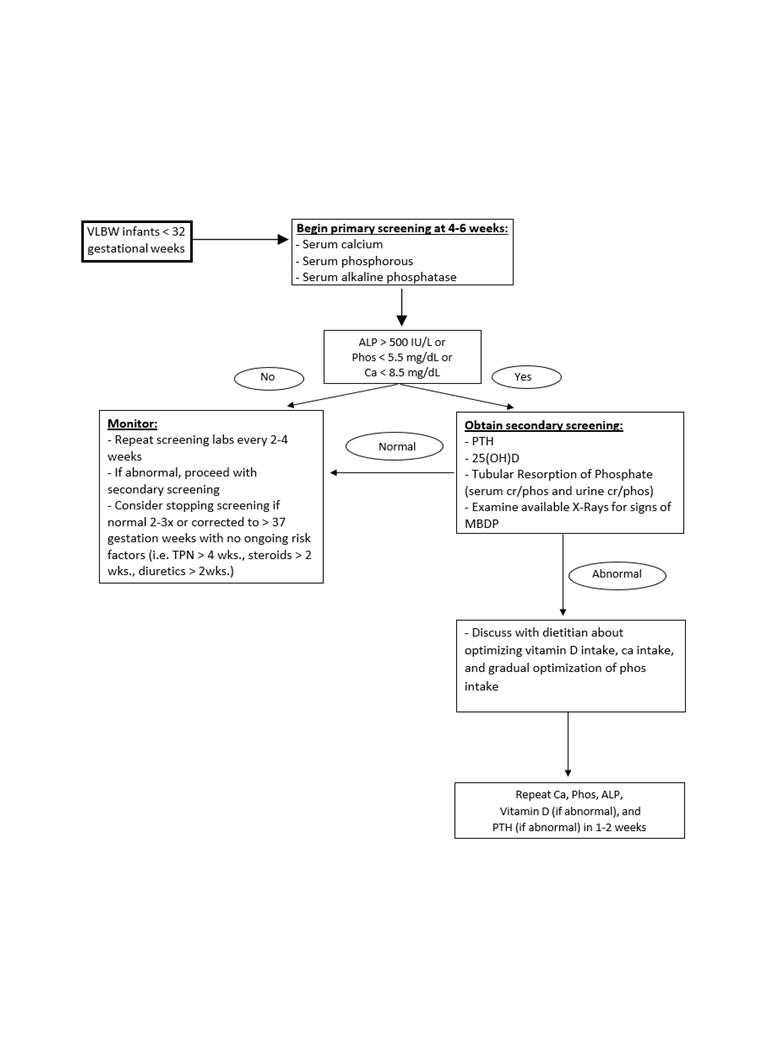
Design/Methods: This is a pre-/post-intervention quasi-experimental design study using quality improvement (QI) framework. The baseline period is between July 1, 2020 and June 30, 2021. Our study period is from July 1, 2021 and onward. We identified stakeholders and formed an interdisciplinary team of neonatal providers, dietitians, and nurses. A key intervention was the development and dissemination of a standardized, evidence-based, and tiered screening algorithm for our NICUs (Figure 1). This algorithm along with other interventions were implemented through reiterative Plan-Do-Study-Act (PDSA) cycles starting in July 2021.
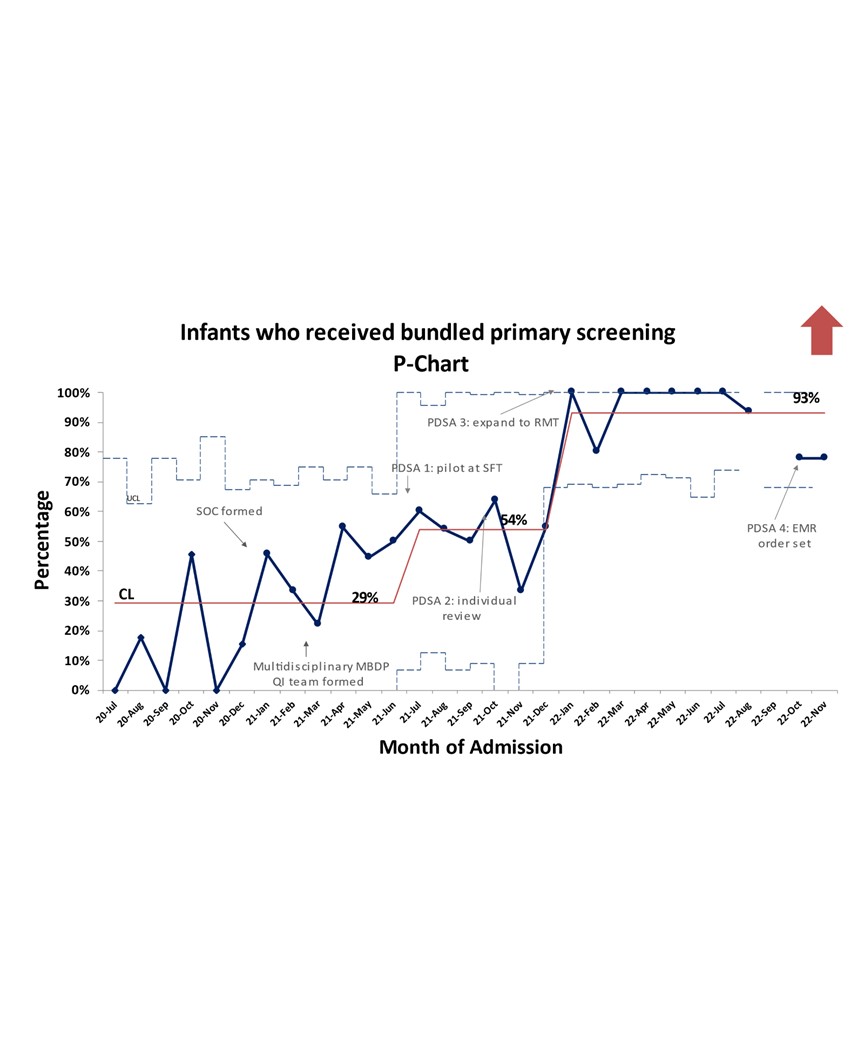
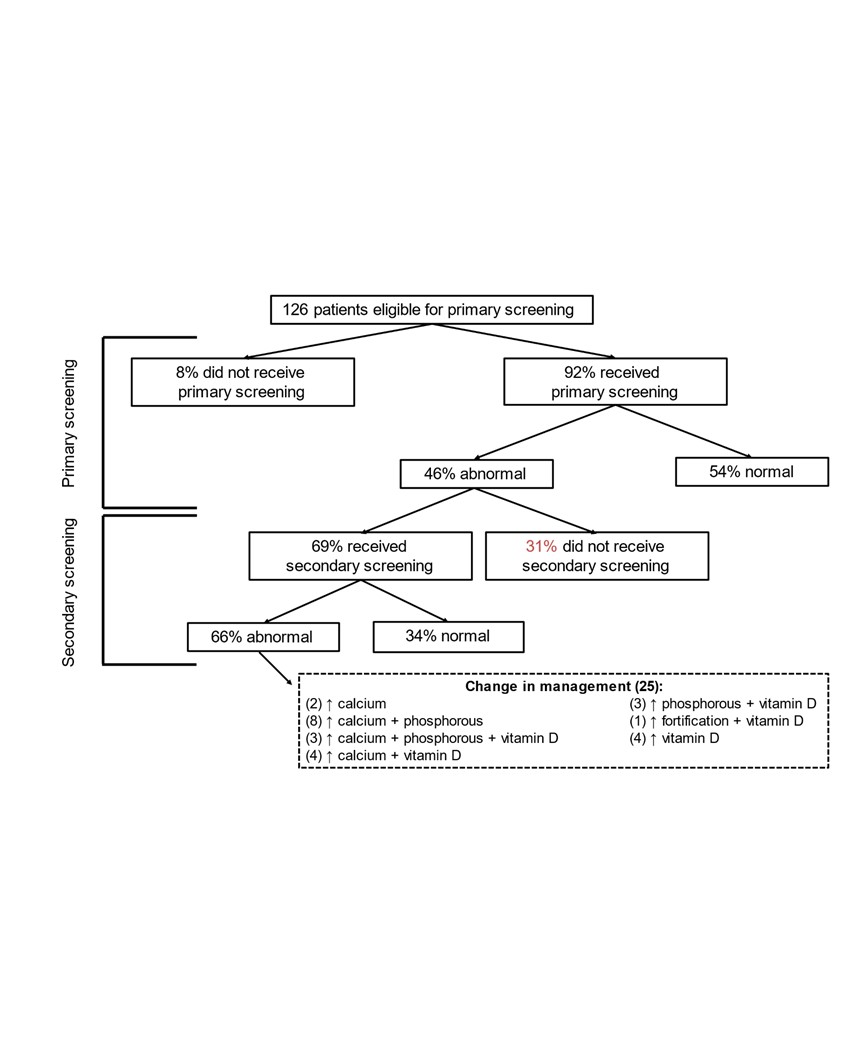
Results: A total of 129 patients were included in the baseline period and 164 patients so far in the post-intervention period. Our baseline proportion of infants who received bundled primary screening was 29% and we were able to move our centerline to 93% with intervention (Figure 2). Abnormal results were seen with primary screening in 46% of infants (Figure 3). In the end, a total of 25 of the 126 patients eligible for primary screening (20%) had abnormal primary and secondary screening results that prompted a change in their nutritional management during their hospitalization.
Conclusion(s): Our interdisciplinary QI approach toward MBDP screening has not only resulted in a significant improvement in our primary screening rate but it has also allowed us to optimize supplementation for patients with MBDP. Furthermore, increases in supplementation were unique and individualized to each patient’s deficiencies as determined by their screening results. We plan to sustain the achievements of this QI initiative with primary screening but recognize there is an opportunity to improve secondary screening rates. Our next PDSA cycles will focus on helping us improve our secondary screening rate.