Pharmacy and Therapeutics: Medication Quality Improvement
Pediatric Therapeutics and Pharmacology
773 - Clinical variables to predict neonates that would benefit from preemptive pharmacogenetic testing
Publication Number: 773.148
- KG
Katie Gallaway, MPH (she/her/hers)
Research Analyst
Indiana University School of Medicine
Indianapolis, Indiana, United States
Presenting Author(s)
Background:
Neonates requiring neonatal intensive care unit (NICU) care are exposed to many drugs in the NICU and subsequent admissions. Many of these drugs have dosing guidelines from the Clinical Pharmacogenetics (PGx) Implementation Consortium (CPIC) and can be used to personalize drug selection and dosing using patient genetics.
Objective: The aim of this study was to determine the number of drugs prescribed to NICU patients with CPIC guidelines and determine factors that can predict high utilization of these drugs.
Design/Methods:
A retrospective observational study was conducted at an academic children’s hospital. Patients were eligible if admitted to the NICU from 2005–2018 with at least one follow-up encounter within 5 years of NICU admission and received at least one CPIC drug. Electronic medical records were queried for all health system encounters, all drug data, congenital diagnoses, gestational age, and birth weight. Data were analyzed for descriptive statistics and non-parametric tests used to analyze clinical factors that may predict high utilization for drugs with CPIC guidelines.
Results:
During the study period, 4196 patients met inclusion criteria. Drugs/drug classes prescribed, age at prescription, number of readmits, and NICU length of stay are summarized in Table 1. Gestational age, birth weight, and diagnoses were available for 2423 patients. Of these, 72% patients were preterm (< 28-37 weeks) and 66.9% were below normal birth weight (< 2499g). There was a significant relationship between extremely preterm (< 28 weeks) and term ( >37 weeks) neonates, (p< 0.05) and between extremely low birth weight (< 1000g) and normal birth weight (> 2500g), (p< 0.05) in the number of drugs received. There was a significant relationship between all congenital diagnoses and number of drugs received, (p< 0.001), Table 2
Conclusion(s):
Neonates admitted to the NICU are high utilizers of drugs with PGx guidelines. We have shown prematurity, low birth weight, and congenital diagnoses are associated with higher unitization of CPIC guideline drugs compared to term, normal birth weight, and neonates without a congenital disease. Preemptive PGx testing in these high-risk patients could provide personalized drug selection and dosing to help prevent poor outcomes associated with adverse drug reactions throughout the lifetime of the patient. 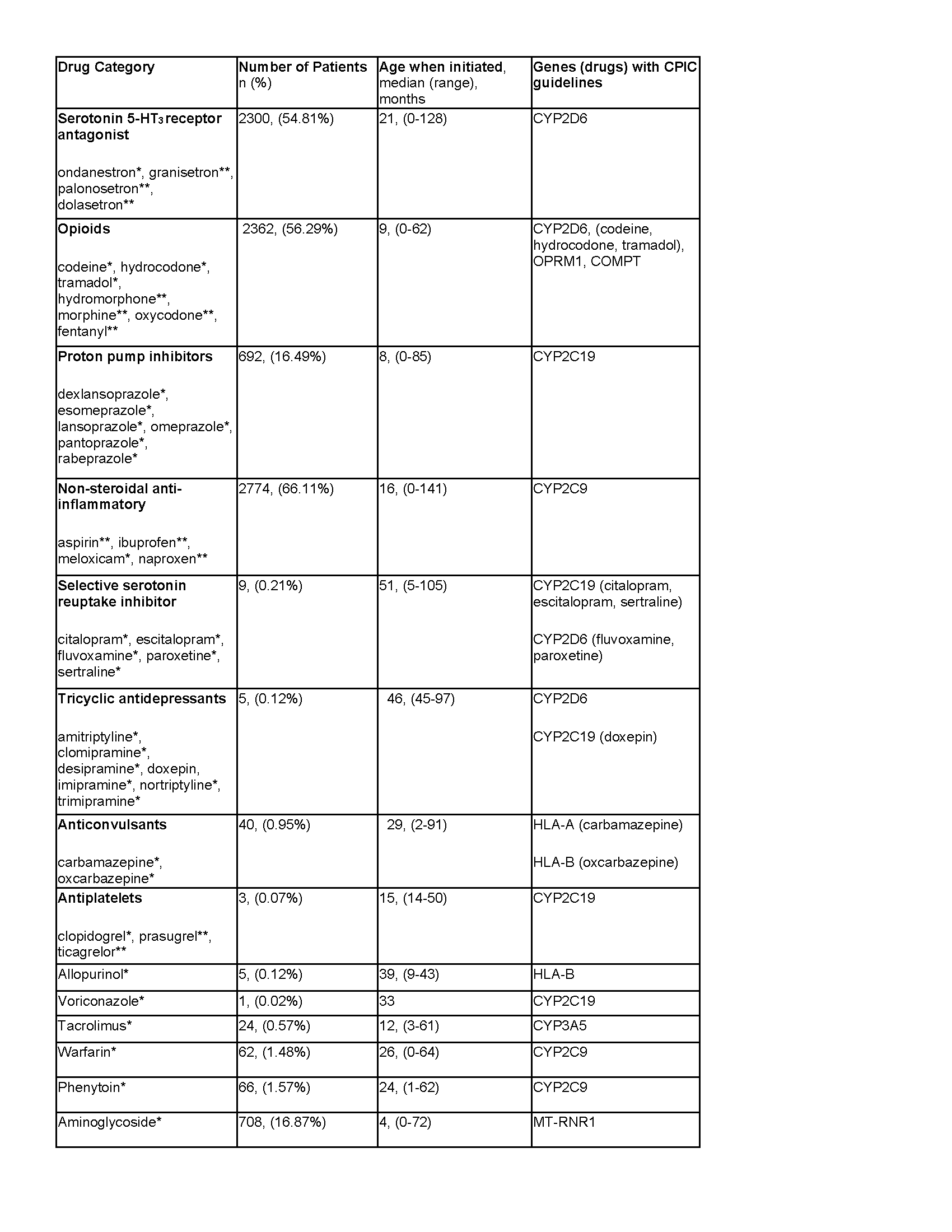
.png)
