Children with Chronic Conditions
Children with Chronic Conditions 1
712 - A randomized controlled trial comparing the effectiveness of a novel central line securement device to traditional securement methods in pediatric patients
Publication Number: 712.105

Ryan St. Pierre-Hetz, MD (he/him/his)
Pediatric Emergency Medicine Fellow
UPMC Childrens Hospital of Pittsburgh
Pittsburgh, Pennsylvania, United States
Presenting Author(s)
Background: Pediatric Central Lines are essential for chronic nutrition and/or medication delivery, but pose a significant risk for mechanical trauma, requiring frequent ER visits and hospitalizations. Additionally, patients have a 33% higher likelihood of line related sepsis in the month following a central line repair. A novel central line securement device was created with the goal of protecting central lines from mechanical trauma. This device has been used by patients for 5 years, however its effectiveness has not been evaluated to date.
Objective: The objective of this pilot study was to investigate if mechanical central line complications decrease after the use of the novel securement device compared prior to its use. The primary outcomes were rates of line trauma (breaks or dislodgements requiring repair), line replacements, or line infections.
Design/Methods:
Of 37 eligible patients who use a central line for intestinal failure, 23 patients were enrolled. 12 patients were randomized to receive a novel securement device in addition to a sterile dressing and 11 patients were randomized to continue using a sterile dressing alone. We compared the rate of complications (rate/month) for the year before and the year after the use of the respective securement devices for each group (novel device or sterile dressing). Patients were followed for up to one year, or for the duration of wearing the device. One patient from each group was lost to follow up. Data regarding line complications were extracted from the medical record. The rate of events per month were compared pre- and post-intervention for each group using a Paired T-Test.
Results:
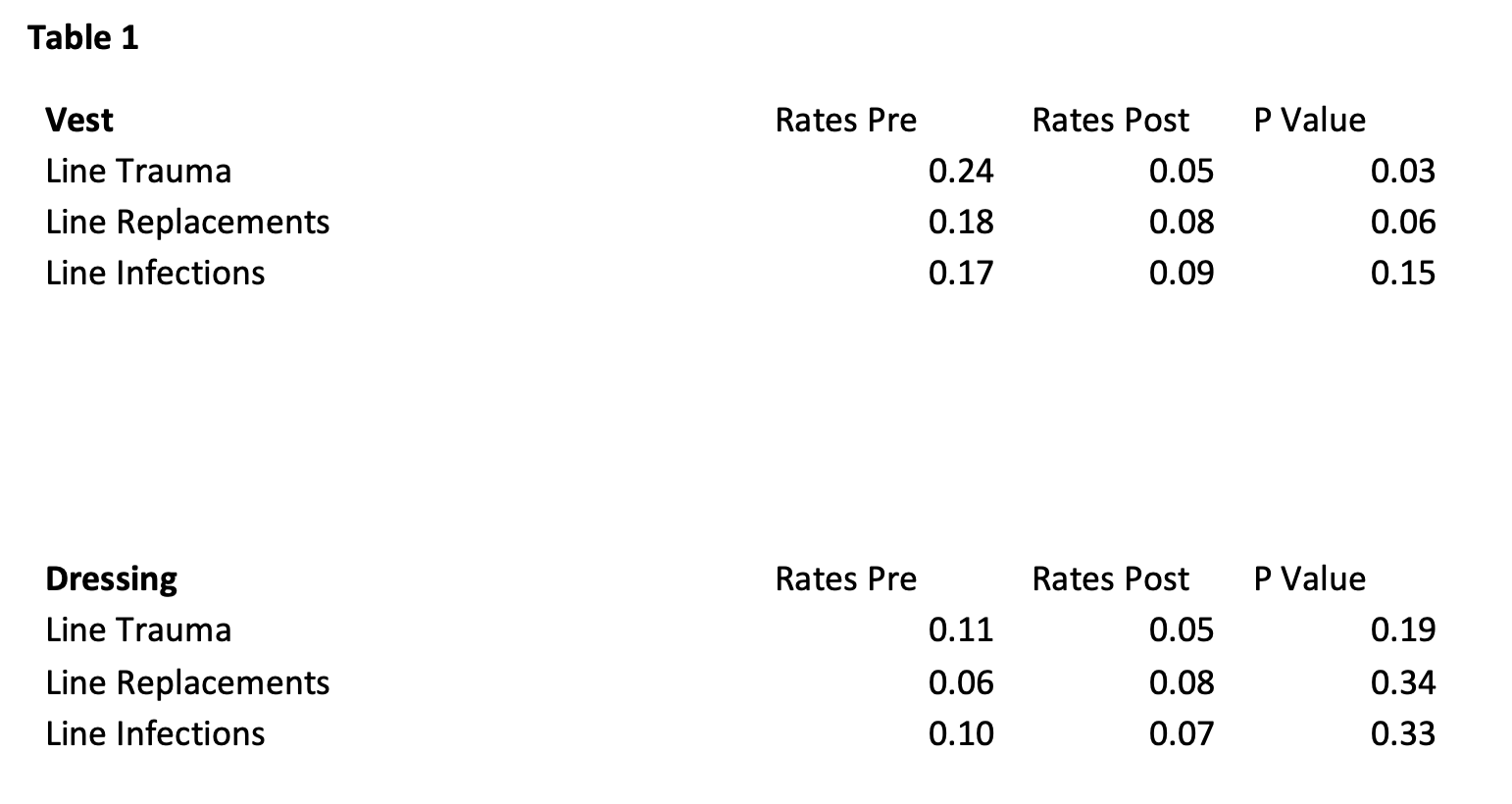
In the 11 patients wearing a novel securement device there was a rate of 0.2 for line trauma/mo prior to enrollment and 0.05/mo after enrollment (p=0.03). There was a rate of 0.18 line replacements/mo prior to enrollment and 0.08/mo after enrollment (p=0.06). Lastly, there was a rate of 0.2 line infections/mo prior to enrollment and 0.09/mo after enrollment (p=0.1). Our 10 control patients had a rate of 0.1 for line trauma/mo prior to enrollment and 0.05/mo after enrollment (p=0.1). There was a rate of 0.06 line replacements/mo prior to enrollment and 0.08/mo after enrollment (p=0.3). Lastly, there was a rate of 0.1 line infections/mo prior to enrollment and 0.07/mo after enrollment (p=0.3). (Table 1)
Conclusion(s):
In this pilot study, we found that the number of line breaks and dislodgements decreased in patients wearing the novel securement device, but not in patients using the sterile dressing alone. The effectiveness of this device should be evaluated in larger studies.