Breastfeeding/Human Milk
Breastfeeding/Human Milk 3: Human Milk Bioactives and Composition
17 - Maternal Immunization during the Second Trimester of Pregnancy Induces a Robust IgA Response in Human Milk
Publication Number: 17.201

Ronit Lubetzky, MD (she/her/hers)
Director, Department of Pediatrics
Dana Dwek Children's Hospital, Tel Aviv Medical Center
Tel Aviv, Tel Aviv, Israel
Presenting Author(s)
Background: Antibody response in human milk (HM) following maternal immunization with BNT162b2 mRNA vaccine is important for neonate protection during early infancy.
Objective:
We aimed to evaluate the antibody response of IgG/IgA/IgM at different lactation stages and to determine the optimal vaccination timing during pregnancy.
Design/Methods:
This is a prospective cohort study conducted in Israel between April 2021 and July 2022. We recruited women post-partum who received the BNT162b2 COVID-19 mRNA-vaccine during the second or the third trimester of pregnancy. HM samples, colostrum (day 0-3), transitional milk (4-14 days), mature milk (above 14 days) were collected post-delivery.
Results:
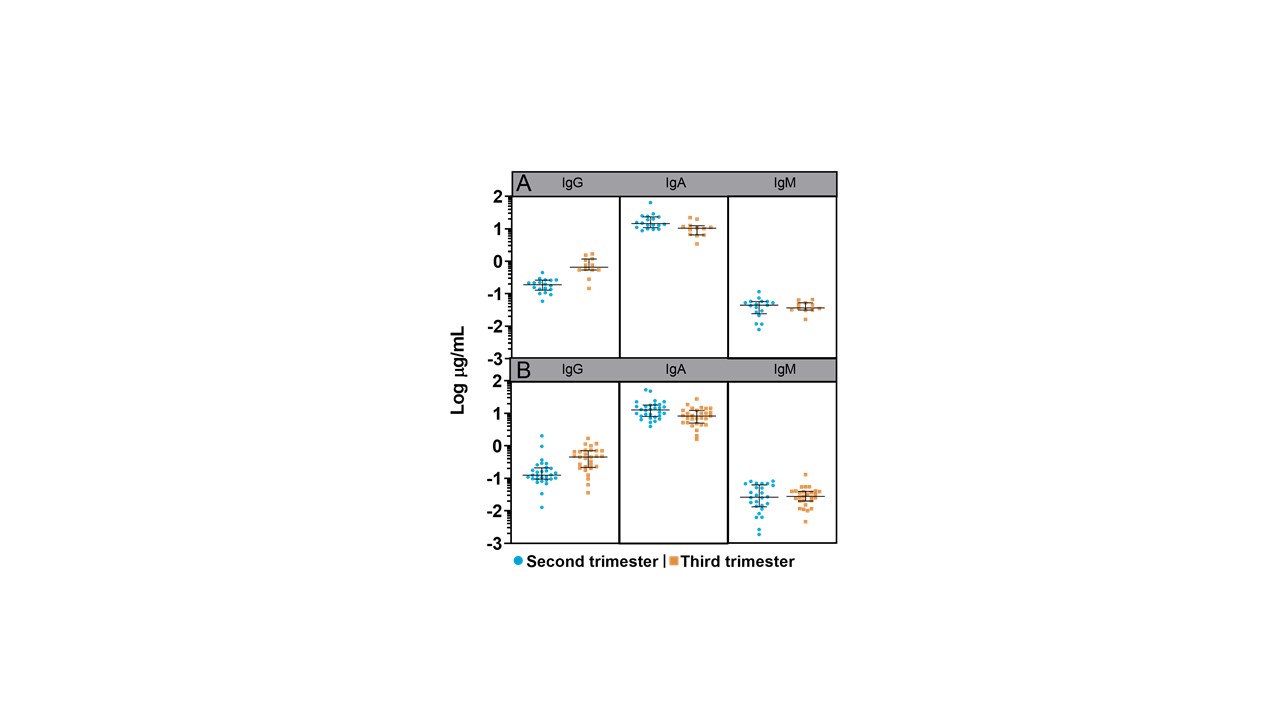
Sixty-two lactating women were included. Late colostrum showed the highest median (IQR) antibody concentration of vaccine-specific IgG (1.098 μg/mL [0.489-2.424], IgA (46.19 μg/mL, [30.89-77.54] and IgM (0.144 μg/mL, [0.089-0.356]). Timing of maternal immunization affected the antibody response in transition and mature milk, (Figure 1). IgA concentrations were the highest of all isotypes in women immunized during the second trimester versus the third trimester in transitional and mature milk (median [IQR], 13.8 μg/mL [10.32-22.3] vs 9.915 μg/mL [6.199-11.82], P = .01 and 13.06 μg/mL [8.232-18.41] vs 8.51 μg/mL [5.125-12.6], P = .006 respectively). IgG levels were higher when immunization occurred during the third trimester versus second trimester in transitional and mature milk (median [IQR] 0.66 μg/mL [0.538-1.167] vs 0.188 μg/mL [0.128-0.261] and 0.451 μg/mL [0.215-0.704] vs 0.125 μg/mL [0.094-0.208], P < .001, respectively).
Conclusion(s):
Our results suggest that maternal immunization with the BNT162b2 mRNA-vaccine during the second trimester of pregnancy provides a higher concentration of vaccine-specific IgA throughout lactation stages.