Nephrology: Dialysis
Nephrology 1: AKI
258 - A combined ex vivo and physiologically based pharmacokinetic approach to determining drug dosing in children on continuous renal replacement therapy
Publication Number: 258.25

Autumn McKnite (she/her/hers)
Graduate Research Fellow
University of Utah
Salt Lake City, Utah, United States
Presenting Author(s)
Background:
Critically ill pediatric patients on continuous renal replacement therapy (CRRT) have high mortality rates ranging from 30-70%, due in part to altered and ineffective drug dosing. Changes in drug exposure can result from 1) underlying disease and 2) direct drug interaction and/or clearance by the CRRT circuit. The extent to which these effects interact and modify drug pharmacokinetics (PK) is currently unknown for most drugs.
Objective: To develop a midazolam physiologically based pharmacokinetic (PBPK) model to translate results from ex vivo drug-circuit interaction studies into bedside dosing recommendations for children on CRRT.
Design/Methods:
Midazolam was injected at therapeutic concentration into ex vivo CRRT circuits and concentrations measured over a 6-hour period. A control was paired with each circuit to determine the amount of drug degradation. A previously published adult PBPK midazolam model was scaled to children. A compartment representing the CRRT circuit was created, parameterized using ex vivo data, and added to the pediatric PBPK model. The midazolam pediatric CRRT-PBPK model was used to predict drug exposure in two children of varying ages undergoing different CRRT modalities. Observed data from an ongoing opportunistic PK study of drugs in children on CRRT were used to validate CRRT-PBPK models. Average fold error (AFE) and absolute average fold error (AAFE) were used to determine model bias and precision.
Results: Midazolam ex vivo mean circuit recovery was 48% at 6 hours with no degradation present in the control (Fig. 1). The initial CRRT-PBPK model underpredicted concentrations from the two study participants (Fig. 2A). Adult and pediatric patients on CRRT commonly experience low albumin and hematocrit. These parameters were subsequently adjusted to reflect changes due to disease state, improving model simulations (Fig. 2A). Final model performance was good with all patient samples within the 90% confidence interval and AFE values of 0.63 and 1.24, and AAFE of 1.58 and 1.78 (Fig. 2A, B).
Conclusion(s): The midazolam CRRT-PBPK model simulated drug concentrations in critically ill pediatric patients undergoing different modalities of CRRT. The results highlight the need to include accurate representation of disease state. This is the first PBPK model to incorporate a CRRT specific compartment and accurately represent drug concentrations in this patient population. This combined ex vivo, CRRT-PBPK modeling approach can be used for other drugs to simulate concentrations in children on CRRT and guide dosing.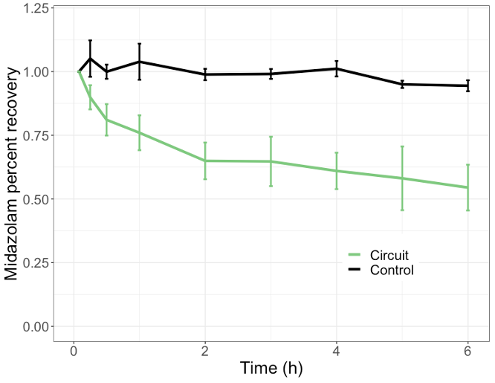
.png)
