Neonatal General
Neonatal General 4: GI-Nutrition-Growth
688 - Assessing the clinical impact of supplemental human milk cream in very low birth weight infants receiving an exclusive-human based nutrition
Saturday, April 29, 2023
3:30 PM - 6:00 PM ET
Poster Number: 688
Publication Number: 688.232
Publication Number: 688.232
Manas Tetarbe, Keck School of Medicine of the University of Southern California, Glendale, CA, United States; Millie Chang, Children’s Hospital Orange County, Yorba Linda, CA, United States; Lorayne Barton, LAC+USC Medical Center, Los Angeles, CA, United States; Rangasamy Ramanathan, Keck School of Medicine of USC, Los Angeles, CA, United States; Rowena Cayabyab, University of Southern California/LAC+USC Medical Center, Los Angeles, CA, United States

Manas Tetarbe, D.O.
Neonatal-Perinatal Medicine Fellow
Keck School of Medicine of the University of Southern California
Glendale, California, United States
Presenting Author(s)
Background: AAP recommends use of expressed breast milk (EBM) or donor human milk (DHM) in preterm infants fortified with proteins, minerals, and vitamins to ensure optimal nutrient intake. A higher rate of hypoglycemia has been seen in our center after the implementation of an exclusive human milk (EHM) diet in very low birth weight (VLBW) infants. Cream derived from human milk can be used to increase caloric intake for infants with poor weight gain on EHM feeding.
Objective: The objective of this study is to assess the clinical impact of adding human milk cream to an EHM diet in VLBW infants.
Design/Methods: Retrospective study of all VLBW infants admitted to neonatal intensive care unit receiving EHM feeding before and after the implementation of cream supplementation to EHM started at 100 ml/kg/day of enteral feeding from November 2017 - September 2022. Infants in the cream cohort were included in the study if they received > 7 days of cream. Neonatal demographics, growth velocity, laboratory values and clinical outcomes such as severe retinopathy of prematurity (ROP), bronchopulmonary dysplasia (BPD), late-onset sepsis (LOS), were collected. Hypoglycemia was defined as blood glucose < 55 mg/dL within 72 of reaching full enteral feeding and off of TPN or intravenous fluids.
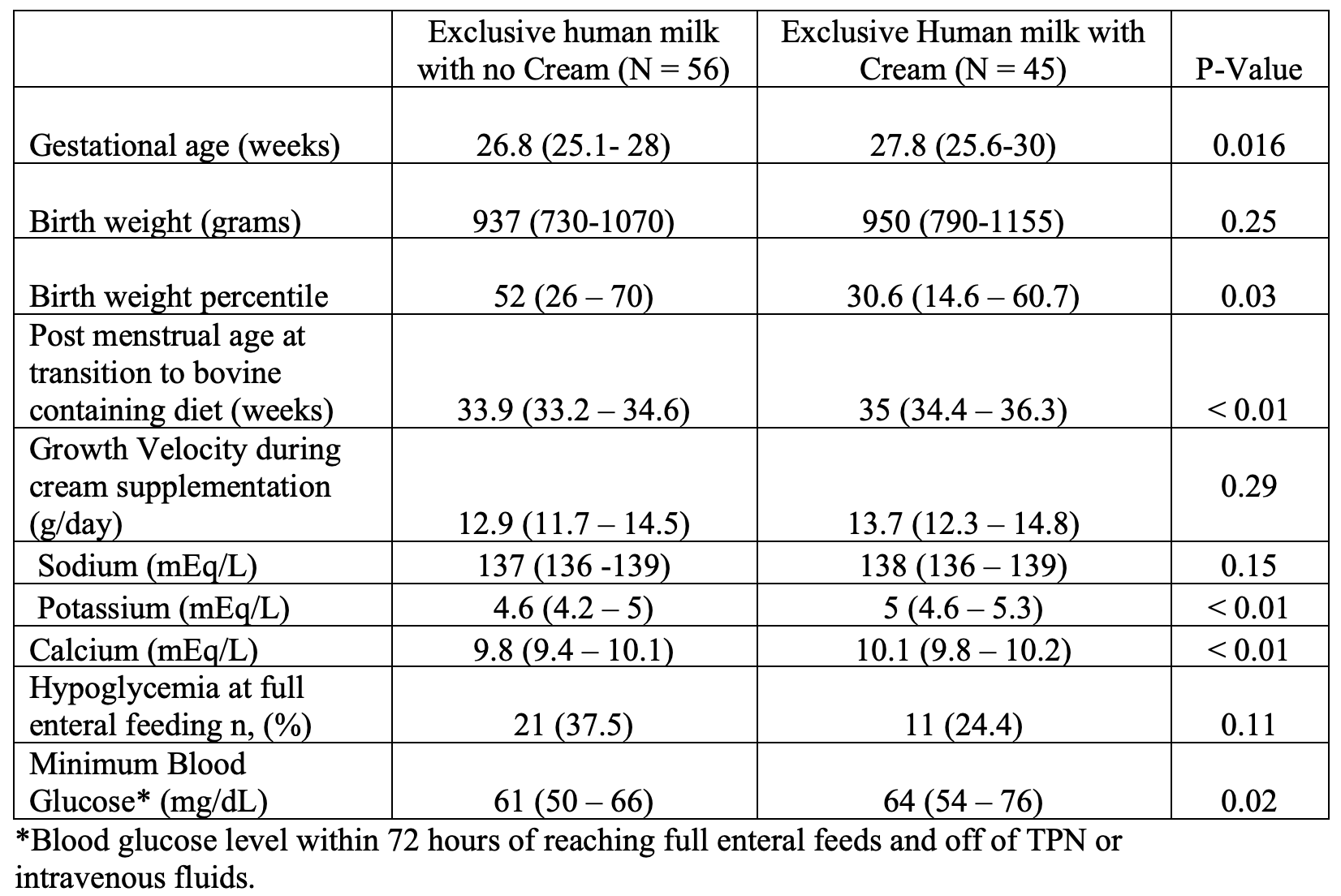
Results: A total of 101 infants were included in the study. 56 received EHM (median birth weight [BW]: 937 g and median gestational age [GA]: 26.8 weeks), and 45 received EHM + Cream (median BW: 950 g, GA: 27.8 weeks). Median duration of cream supplementation was 29 days. There was no significant difference in rates of BPD, ROP, and LOS. The growth velocity in the cream cohort was not significantly higher before transition to a bovine derived diet but the cream cohort had significantly higher median levels of serum potassium and calcium at time of transition to a bovine derived diet. Median minimum blood glucose levels were significantly higher in the cream cohort, and incidence of hypoglycemia was lower (37.5% vs 24.4%) but not statistically significant. (Table 1)
Conclusion(s): Our preliminary findings suggest that implementation of cream in VLBW infants led to improved electrolyte measures and blood glucose levels but has a limited impact on rate of hypoglycemia and growth. We hypothesize that hypoglycemia and growth can possibly be improved by the early initiation of cream supplementation but further studies with a larger study population is needed.