Neonatal Nephrology/AKI
Neonatal Nephrology/AKI 2
241 - Dosing Variation in CRRT prescription in Neonates and infants weighing less than 10 kg: An analysis of the Worldwide Exploration of Renal Replacement Outcomes Collaborative in Kidney Disease (WE-ROCK)
Saturday, April 29, 2023
3:30 PM - 6:00 PM ET
Poster Number: 241
Publication Number: 241.243
Publication Number: 241.243
Shina Menon, University of Washington School of Medicine, Seattle, WA, United States; Michaela Collins, Cincinnati Children's Hospital Medical Center, Cincinnati, OH, United States; Mihaela Damian, Stanford University School of Medicine, Palo Alto, CA, United States; Dana Fuhrman, UPMC Childrens Hospital of Pittsburgh, Pittsburgh, PA, United States; Michelle C. Starr, Indiana University School of Medicine, Indinapolis, IN, United States; Tennille Webb, University of Alabama at Birmingham/Children's of Alabama, Birmingham, AL, United States; Kelli A. Krallman, Cincinnati Children's Hospital Medical Center, Cincinnati, OH, United States; Nicholas Ollberding, Cincinnati Children's Hospital Medical Center, Cincinnati, OH, United States; Huaiyu Zang, Cincinnati Children's Hospital Medical Center, Cincinnati, OH, United States; Katja M. Gist, Children's Hospital Medical Center (Cincinnati), Cincinnati, OH, United States

Shina Menon, MD (she/her/hers)
Associate Professor
University of Washington School of Medicine
Seattle, Washington, United States
Presenting Author(s)
Background: Continuous Kidney Replacement Therapy (CKRT) prescriptions in neonates, infants and children are extrapolated from adults, and there exist wide variations in clinical practice. Some pediatric centers dose CKRT in mL/kg/hr, and others normalize to body surface area (BSA) of 1.73m2. In adults, a prescribed dose of 25-30 mL/kg/hr is recommended, which typically approximates to 2000 mL/hr/1.73m2. In infants and neonates, the nonlinear relationship between weight and BSA may result in a higher prescribed dose.
Objective: We aimed to evaluate prescription variance in neonatal and infant CKRT prescribing patterns, and if a higher dialysis dose is associated with worse outcomes
Design/Methods: The Worldwide Exploration of Renal Replacement Outcomes Collaborative in Kidney Disease (WE-ROCK) study is a retrospective international multicenter study (32 centers, 7 nations) of patients aged 0-25 years treated with CKRT for Acute Kidney Injury (AKI) or Fluid Overload from 2018-2021. Patients with previous dialysis dependence, ECMO utilization, or who received CRRT for non-AKI/fluid overload indication were excluded. For this analysis, neonates and children weighing < 10 kg at hospital admission were assessed
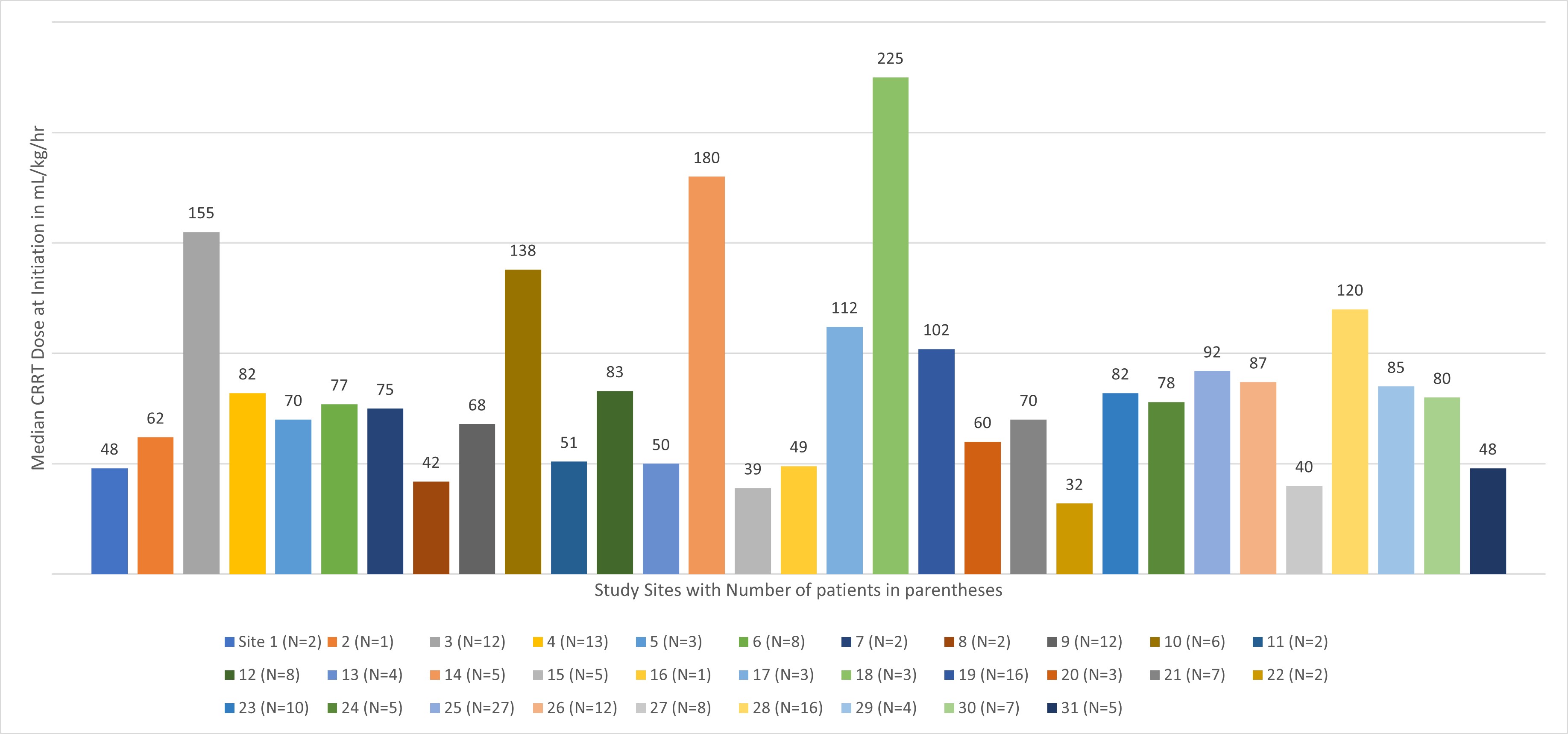
Results: Data from 214 infants were included (43% female), median (IQR) age of 0.53 (0.10, 0.95) years. Median (IQR) CKRT dose was 2107.12 ml/1.72m2/hr (1569.31, 3078.72) or 63.85 ml/kg/hr (49.93, 88.73). Most patients (n=179, 84%) received a dose > 40 mL/kg/hr. 115 (54%) survived to ICU discharge. There was no significant difference in median (IQR) CKRT dose at initiation between survivors and non survivors [60.00 (49.95, 85.78) mL/kg/hr vs 69.93 (49.30, 101.88) mL/kg/hr, p=0.2]. The prescribed dose increased over time and by Day 4 was significantly different between survivors and non survivors [63.59 (43.29, 90.80) mL/kg/hr vs 82.99 (55.19, 114.61) mL/kg/hr, p=0.02]. MAKE-90 (composite outcome of death, new dialysis, or worsened kidney function at 90 days) occurred in 152 (71%). There were no differences in CKRT dose between those with and without MAKE-90. There was significant variation in CKRT dosing across centers ranging from 32 mL/kg/hr to 225 mL/kg/hr (Figure 1)
Conclusion(s): This is the largest report of neonates and infants receiving CKRT. There is lack of standardized CKRT dosing across centers with neonates and infants receiving higher doses than what is recommended by KDIGO. It is unclear if higher dose negatively impacts outcomes. Prospective studies evaluating the prescribed and delivered dose are needed