Neonatal Fetal Nutrition & Metabolism
Neonatal GI Physiology & NEC 4: Gut Health, Enteral Nutrition and Oral Feeding
79 - Effects of Prophylactic Probiotics Supplementation on Infants Born Very Preterm or Very Low Birth Weight
Saturday, April 29, 2023
3:30 PM - 6:00 PM ET
Poster Number: 79
Publication Number: 79.236
Publication Number: 79.236
Rachael A. Hanson, Texas A&M Health Science Center College of Medicine, Dallas, TX, United States; Heather S. Hendrikson, Baylor Scott and White Health, BUMC Dallas, Flower Mound, TX, United States; Mustafa Suterwala, Pediatrix Medical Group and Baylor University Medical Center, Dallas, TX, United States; Jordan Reis, Baylor Scott & White Dallas, Dallas, TX, United States; Sujata Desai, Baylor University Medical Center, Dallas, TX, United States; Arpitha Chiruvolu, Baylor University Medical Center, Dallas, TX, United States

Rachael A. Hanson (she/her/hers)
MS3
Texas A&M Health Science Center School of Medicine
Dallas, Texas, United States
Presenting Author(s)
Background: As dysbiosis appears to be an important risk factor for the development of necrotizing enterocolitis (NEC) in very preterm (VP) infants, optimizing the preterm gut microbiome with probiotics is a favorable strategy to prevent NEC. Although, multiple previous meta-analyses have reported a reduction of NEC, the heterogeneity in study methods and uncertainty regarding efficacy of different probiotic preparations make it difficult to generalize the results and this is reflected in the limited current routine use of probiotics in neonatal intensive care units (NICUs) in the United States.
Objective: To evaluate the effects of a multi-strain NICU-specific probiotic product (Similac Triblend™ consisting of Bifidobacterium lactis (BB-12®), Bifidobacterium infantis (BB-02™) and Streptococcus thermophilis (TH-4®)) on infants born VP or very low birth weight (VLBW). We hypothesized that a guideline-driven supplementation would be associated with a reduction of NEC incidence by 47%, from the baseline of 6.3% to 3.3% in one year after implementation based on reported effect estimates (Sawh SC, et al. Prevention of necrotizing enterocolitis with probiotics: a systematic review and meta-analysis. PeerJ. 2016;4:e2429).
Design/Methods: In this cohort study, 125 infants born VP or VLBW who received probiotics during the prospective study period (one year after implementation; July 1, 2020 to June 30, 2021; Probiotics group) were compared to a retrospective cohort of 126 VP or VLBW infants who qualified to receive probiotics, but did not, as they were born one year prior to implementation (July 1, 2019 to June 30, 2020; No probiotics group). The outcomes of interest were NEC, death and late-onset sepsis.
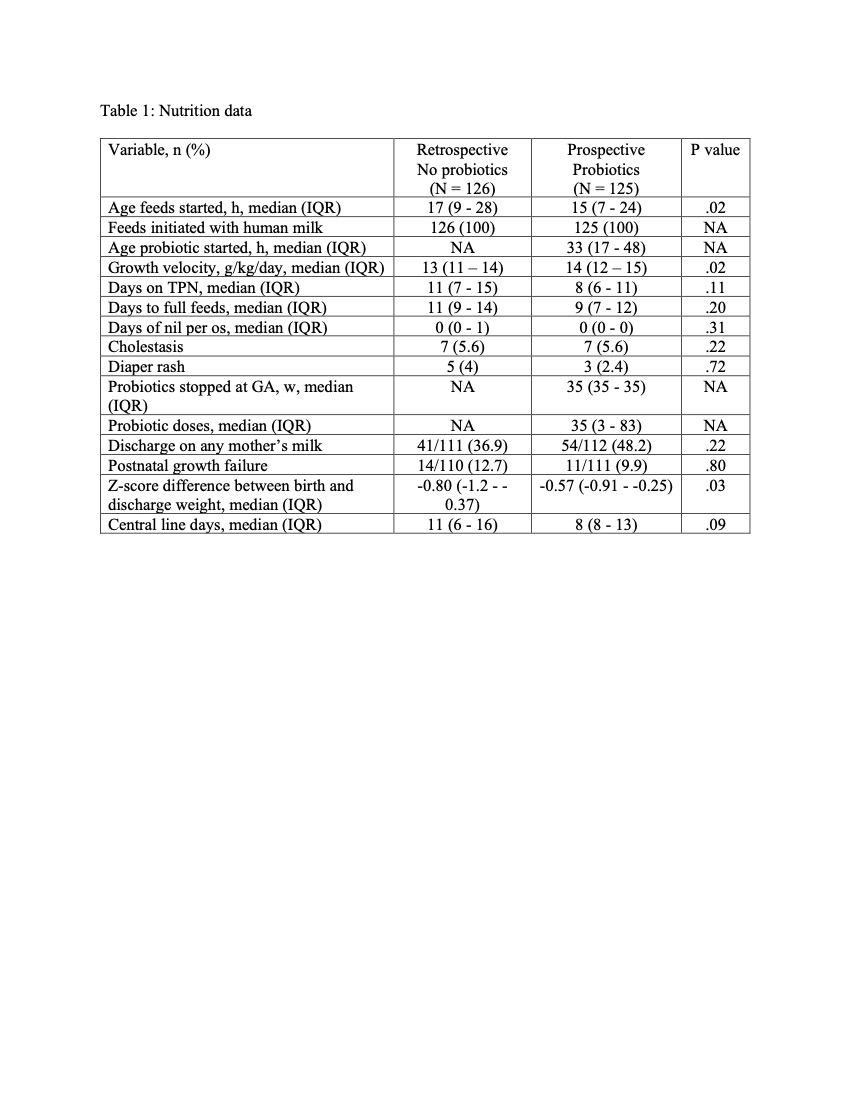
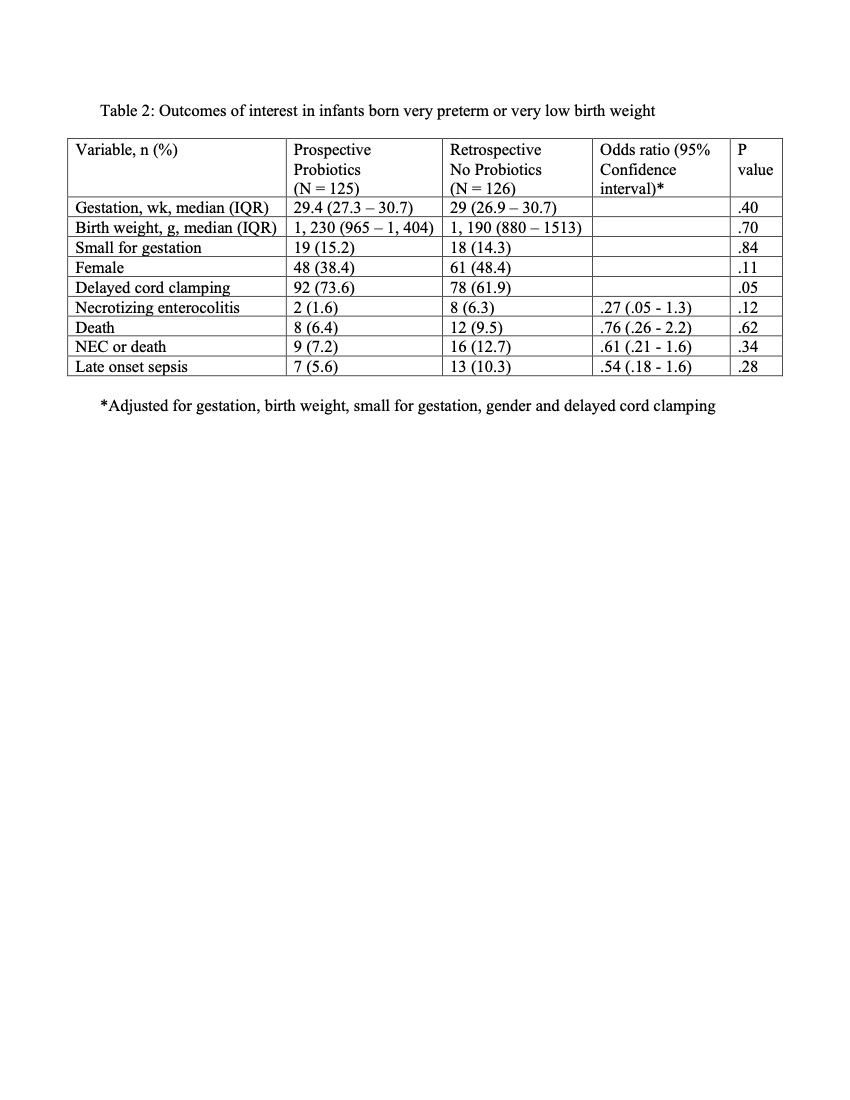
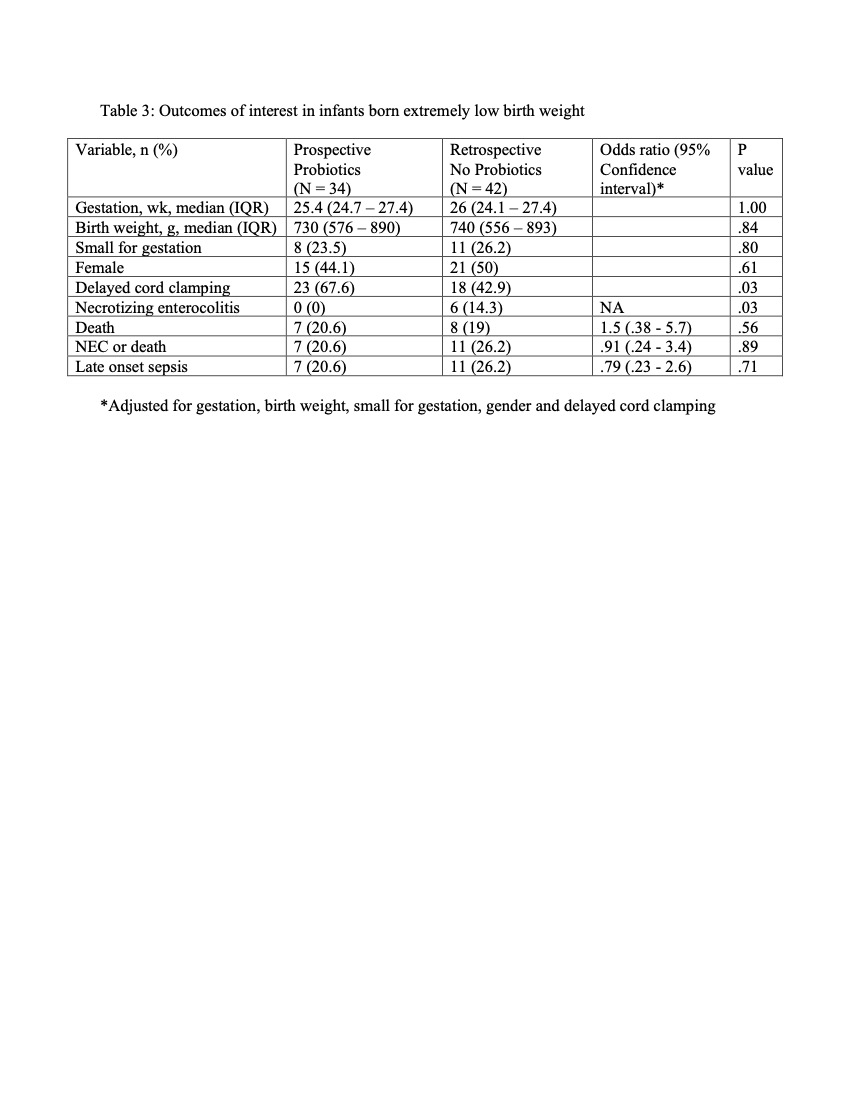
Results: The median age of starting probiotics was 33 hours of life and were given until 35 weeks post menstrual age. Compared to No probiotics group, the growth velocity was significantly higher in the Probiotics group (14 vs 13 g/kg/day). (Table 1). The incidence of NEC decreased from 6.3% to 1.6% (a risk reduction of 75%). Multiple logistic regression after adjusting for different variables showed no significant differences in the outcomes of interest. (Table 2) In the subset of infants born extremely low birth weight, the incidence of NEC was significantly lower in the Probiotics group compared to No probiotics group (0% vs 14.3%). (Table 3) No adverse effects were observed.
Conclusion(s): Although nonsignificant, routine probiotics supplementation in infants born VP or VLBW resulted in a reduction of NEC which met the literature reported effect estimates.