Neonatal Infectious Diseases/Immunology
Neonatal Infectious Diseases/Immunology 2
674 - The updated “Triple I” classification and guidelines lead to a decreased incidence of clinical chorioamnionitis and antibiotic use in neonates
Saturday, April 29, 2023
3:30 PM - 6:00 PM ET
Poster Number: 674
Publication Number: 674.241
Publication Number: 674.241
Amy J. Sloane, Nemours/Sidney Kimmel Medical College at Thomas Jefferson University, Dresher, PA, United States; Karen Leopold, Nemours Children's Hospital, Garnet Valley, PA, United States; Lisa Madden-Schmidt, Thomas Jefferson University Hospital, Mullica Hill, NJ, United States; Margaret Lafferty, Nemours Children's Hospital, Philadelphia, PA, United States; David Carola, Sidney Kimmel Medical College at Thomas Jefferson University, Philadelphia, PA, United States; Kolawole O. Solarin, NemoursAlfred I. duPont Hospital for Children/Thomas Jefferson University Hospital, Philadelphia, PA, United States; Zubair Aghai, Sidney Kimmel Medical College at Thomas Jefferson University, Philadelphia, PA, United States

Amy J. Sloane, MD, MHS (she/her/hers)
Neonatologist
Nemours Children’s Health / Thomas Jefferson University Hospital
Dresher, Pennsylvania, United States
Presenting Author(s)
Background: In 2016, the National Institute of Child Health and Human Development (NICHD) recommended to replace the term chorioamnionitis with “intrauterine inflammation or infection or both,” or “Triple I”. In 2018, the Committee on the Fetus and Newborn published new guidelines for managing infants at risk for early onset sepsis (EOS). In our institution, we incorporated these recommendations and developed our own guidelines for the management of chorioamnionitis-exposed infants in August 2019.
Objective: To evaluate the impact of the revised guidelines on the incidence of chorioamnionitis during labor and empiric antibiotics and laboratory testing in neonates.
Design/Methods: This is a retrospective study of neonates born ≥35 weeks’ gestation between January 2016-July 2022 to mothers with clinical chorioamnionitis or Triple I. The two cohorts of neonates born before and after the implementation of the revised guidelines were compared. Previously, all chorioamnionitis or Triple I-exposed neonates were admitted to the NICU, had screening CBCs and blood cultures, and were treated with empiric antibiotics. After the new guidelines, neonates were admitted to the NICU for observation and blood culture, but antibiotics were only started for clinical illness or elevated EOS score ( >3/1,000) as determined by the use of the Kaiser Permanente EOS calculator.
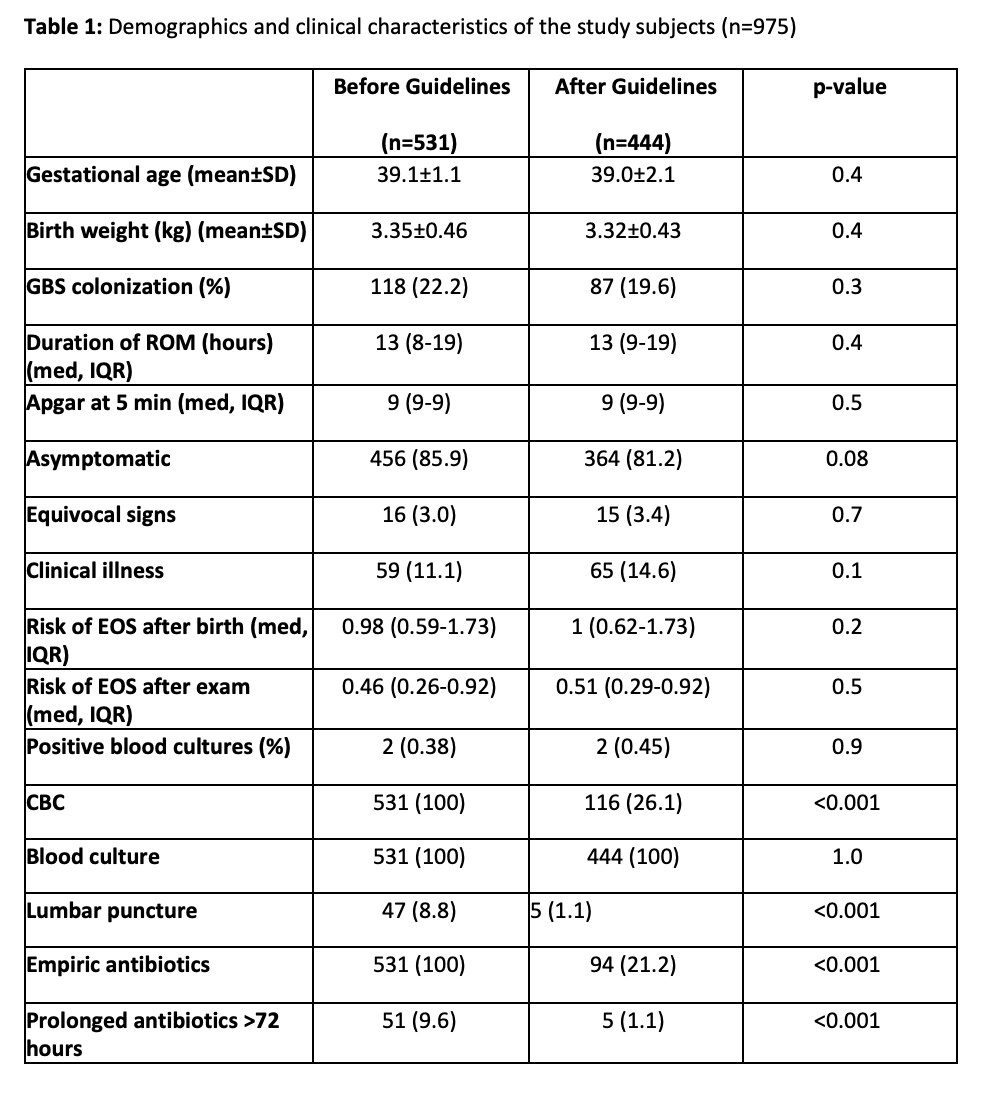
Results: Prior to August 2019, 6,956 infants were delivered, 531 (7.6%) of whom were chorioamnionitis-exposed. After August 2019, 8,608 infants were delivered, 444 (5.2%) of whom were Triple I-exposed. This was a significant decrease in the number of infants admitted for EOS evaluation (p< 0.001). Demographics and EOS calculator scores between cohorts were not significantly different (Table 1). Clinical signs of EOS were identified in 59 (11.1%) infants born to mothers with chorioamnionitis and in 65 (14.6%) of infants born to mothers with Triple I (p=0.08). Only two infants in each cohort (0.38% and 0.45%, respectively) had culture-positive sepsis (p=0.9). All infants had a blood culture sent on admission, but after August 2019 a significantly lower number had CBCs (100% vs 26.1%, p< 0.001) and lumbar punctures (8.8% vs 1.1%, p< 0.001) performed. Empiric antibiotics were decreased significantly from 100% to 21.2% (p< 0.001).
Conclusion(s): The updated guidelines led to a significant decrease in the number of women diagnosed with chorioamnionitis during labor and to the number of infants admitted to the NICU for EOS evaluation. The new guidelines also reduced laboratory testing and use of empiric antibiotics. There were no missed cases of culture-positive EOS.