Asthma
Asthma 2
371 - Impact of Patient Demographic and Disease Characteristics on the Efficacy of Dupilumab in Children with Asthma in the VOYAGE Study
Publication Number: 371.303

Nicholas M. Jellots, PharmD, BCPS (he/him/his)
Medical Science Liaison
Sanofi
Indiana, Pennsylvania, United States
Presenting Author(s)
Background:
Dupilumab is a fully human monoclonal antibody that blocks the shared receptor component of interleukin‑4 and interleukin-13, key and central drivers of type 2 inflammation in multiple diseases. In VOYAGE, a phase 3 study (NCT02948959), dupilumab showed significant reductions in asthma exacerbations, and improvements in lung function in children aged 6 to < 12 years with uncontrolled, moderate-to-severe type 2 asthma (baseline blood eosinophils ≥ 150 cells/μL or fractional exhaled nitric oxide [FeNO] ≥ 20 parts per billion [ppb]); overall safety was consistent with the known dupilumab safety profile. However, Drug treatment effects can vary by patient demographic and disease characteristics. Dupilumab has previously shown efficacy reducing annualized severe asthma exacerbation rate (AER) across a range of baseline demographics and disease characteristics in adults with type 2 asthma.
Objective:
This post hoc analysis of VOYAGE assessed dupilumab efficacy in pre-specified subgroups of pediatric patient based on baseline demographic and disease characteristics.
Design/Methods:
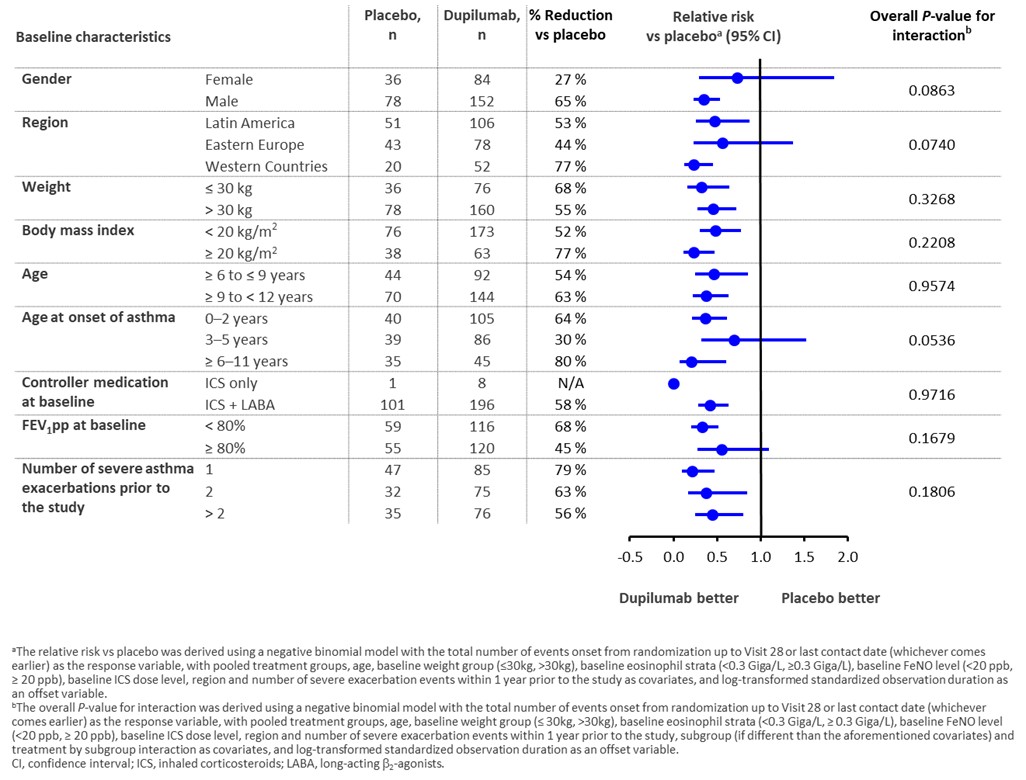
Patients with evidence of type 2 inflammation (baseline eosinophils ≥ 150 cells/μL or FeNO ≥ 20 ppb) were randomized 2:1 to add-on subcutaneous dupilumab 100/200 mg, or matched placebo, every 2 weeks based on body weight for 52 weeks. Primary outcome was AER over the treatment period in each demographic subgroup, stratified by pre-specified baseline demographics of gender, region, weight, body mass index (BMI), and age; and baseline disease characteristics of age at asthma onset, baseline controller medications, % predicted FEV1 (FEV1 pp), and number of severe exacerbations prior to the study.
Results:
Data from a total of 350 patients (dupilumab [n = 236] or placebo [n = 114]) with moderate-to-severe asthma and type 2 inflammation were included in this analysis. Dupilumab was associated with consistent reductions in AER compared with placebo across all subgroups defined by baseline demographics, including weight, BMI, age, and by baseline disease characteristics, including history of acute exacerbations, and background therapy regimen (Figure).
Conclusion(s):
In a population with moderate-to-severe asthma and type 2 inflammation, dupilumab’s efficacy in reducing exacerbations was generally consistent across subgroups defined by baseline demographic and disease characteristics.