Neonatal Quality Improvement
Neonatal Quality Improvement 7
216 - Improving Surfactant Delivery in the Era of Less-Invasive Surfactant Administration
Monday, May 1, 2023
9:30 AM - 11:30 AM ET
Poster Number: 216
Publication Number: 216.441
Publication Number: 216.441
Venkata S. Gupta, Cohen Children's Medical Center, Glen Oaks, NY, United States; Vitaliya Boyar, Cohen Children's Medical Center, new hyde park, NY, United States; Olena Predtechenska, Cohen Children's Medical Center, Bellmore, NY, United States; Regina Spinazzola, Cohen Children's Medical Center, Manhasset, NY, United States; Dalibor Kurepa, Cohen Children's Medical Center, New York, NY, United States; Barry Weinberger, Cohen Children's Medical Center, New Hyde Park, NY, United States; Shahana Perveen, Cohen Children's Medical Center, Mount Sinai, NY, United States

Venkata S. Gupta, MD (he/him/his)
Neonatal Fellow
Cohen Children's Medical Center
Glen Oaks, New York, United States
Presenting Author(s)
Background: Early surfactant improves outcomes for infants with respiratory distress syndrome (RDS), and QI initiatives in our NICUs prior to 2020 had led to > 80% administration of surfactant within 30 minutes of qualifying. Less-invasive surfactant administration by thin catheter (LISA) reduces bronchopulmonary dysplasia (BPD) compared to delivery by endotracheal tube, and video laryngoscope-assisted LISA was introduced as the primary route for surfactant in our NICUs in late 2020. Subsequently, the rate of surfactant administration (by either route) within 30 minutes of qualifying dropped to a median of 25% in January 2021.
Objective: The SMART aim was to administer surfactant to > 80% of infants with RDS within 30 minutes of qualifying by 11/1/22. Infants ≤ 1500 grams birth weight and/or ≤ 32 weeks gestation and < 72 hours of age were included.
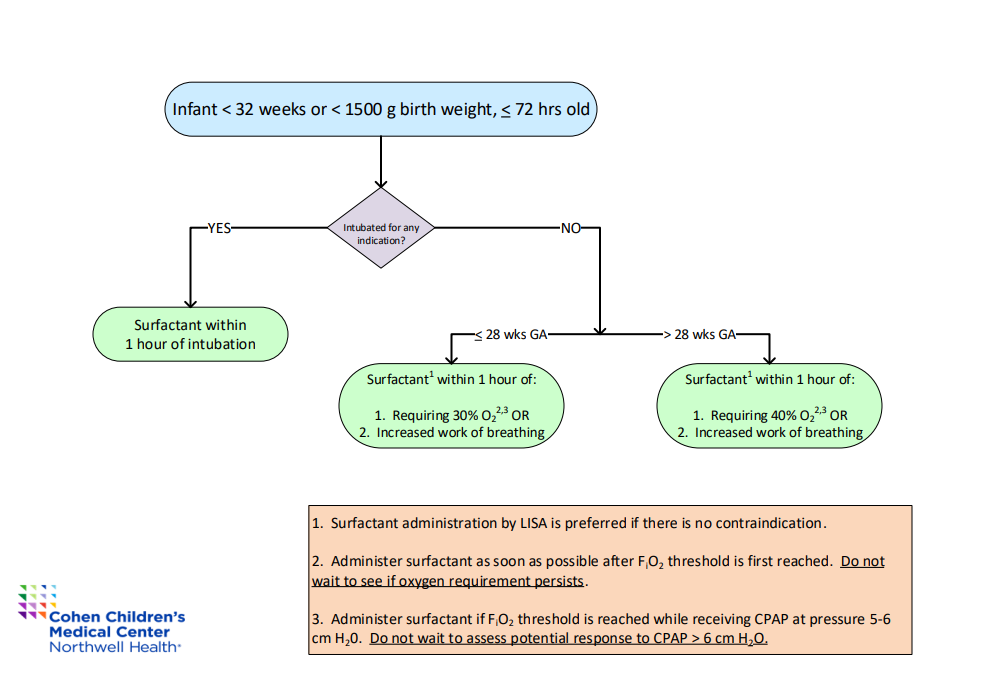
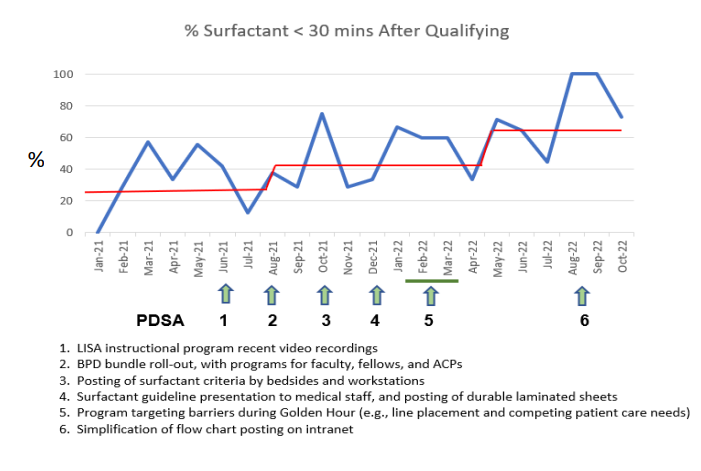
Design/Methods: Surfactant delivery by LISA was implemented in our Level 3 and 4 NICUs as a “high-risk” process starting in late 2020, with extensive protocol development, education, and skill credentialing. Qualification (need for surfactant) was defined as FiO2 > 30% for 1 hour for infants ≤ 28 weeks gestation or > 40% for 1 hour for infants > 28 weeks gestation (Fig 1). Several cycles of change to improve surfactant delivery within those windows are shown in the annotated run chart (Fig 2). These included BPD bundle roll-out, physical and virtual dissemination of the surfactant criteria, and several distinct approaches to instruction. Asymmetric surfactant delivery and air leak were assessed as balancing measures.
Results: The proportion of infants receiving surfactant within 30 minutes of qualifying increased from 25% to 63% (Fig 2). Interventions that improved performance were review of LISA technique using video recordings, incorporation of surfactant guidelines into an inclusive BPD prevention bundle, and a program targeting barriers and prioritization of tasks during the Golden Hour. There were no episodes of asymmetric surfactant delivery or air leak.
Conclusion(s): Introduction of LISA, like any therapeutic innovation, may trigger QI challenges requiring new strategies for maintaining established standards of care. Ongoing instruction on LISA is beneficial, beyond initial provider credentialing. It is also important to provide context (BPD prevention bundle) and to address challenges integrating LISA into the workflow. Although we did not achieve the SMART aim of 80% on-time administration of surfactant, we anticipate that increasing familiarity and skill among the physicians will allow further improvements.