Neonatal Pulmonology
Neonatal Pulmonology 4: Exosomes, Stem Cells, Maternal and Fetal Environmental Effects
138 - IL-17 Mediates Chorioamnionitis-induced Neonatal Chronic Lung Disease
Publication Number: 138.436

Viral G. Jain, MD (he/him/his)
Assistant Professor
University of Alabama School of Medicine
Birmingham, Alabama, United States
Presenting Author(s)
Background: Chorioamnionitis (chorio) is the most common cause of preterm birth. Chorio increases fetal and neonatal mortality as well as bronchopulmonary dysplasia (BPD). The rate of BPD continues to increase for neonates born very preterm, specifically in those exposed to chorio.
Objective:
This study aims to understand the mechanism of development of BPD in chorio-exposed preterm as there are no effective therapies for preventing preterm birth, chorio, or long-term adverse outcomes such as BPD.
Design/Methods:
Chorio was identified by placental histology. Blood (from all infants 22-29 weeks of gestation) for transcriptomic analysis and tracheal aspirate for cytokine analysis (from intubated infants) were collected on postnatal days 1-3. For the mouse model of chorio, pregnant dams at E16.5 were injected with LPS in each amniotic sac at laparotomy, followed by natural birth. Lung samples were collected at specific time points. The lung function of mice was assessed by oscillometry (Flexivent).
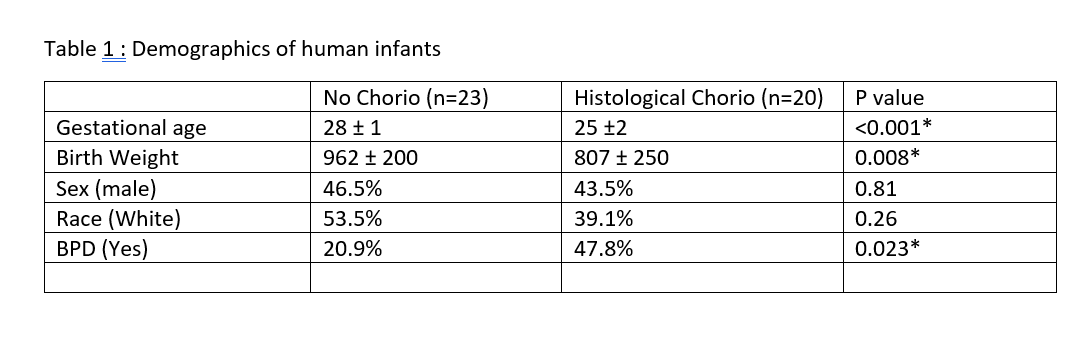
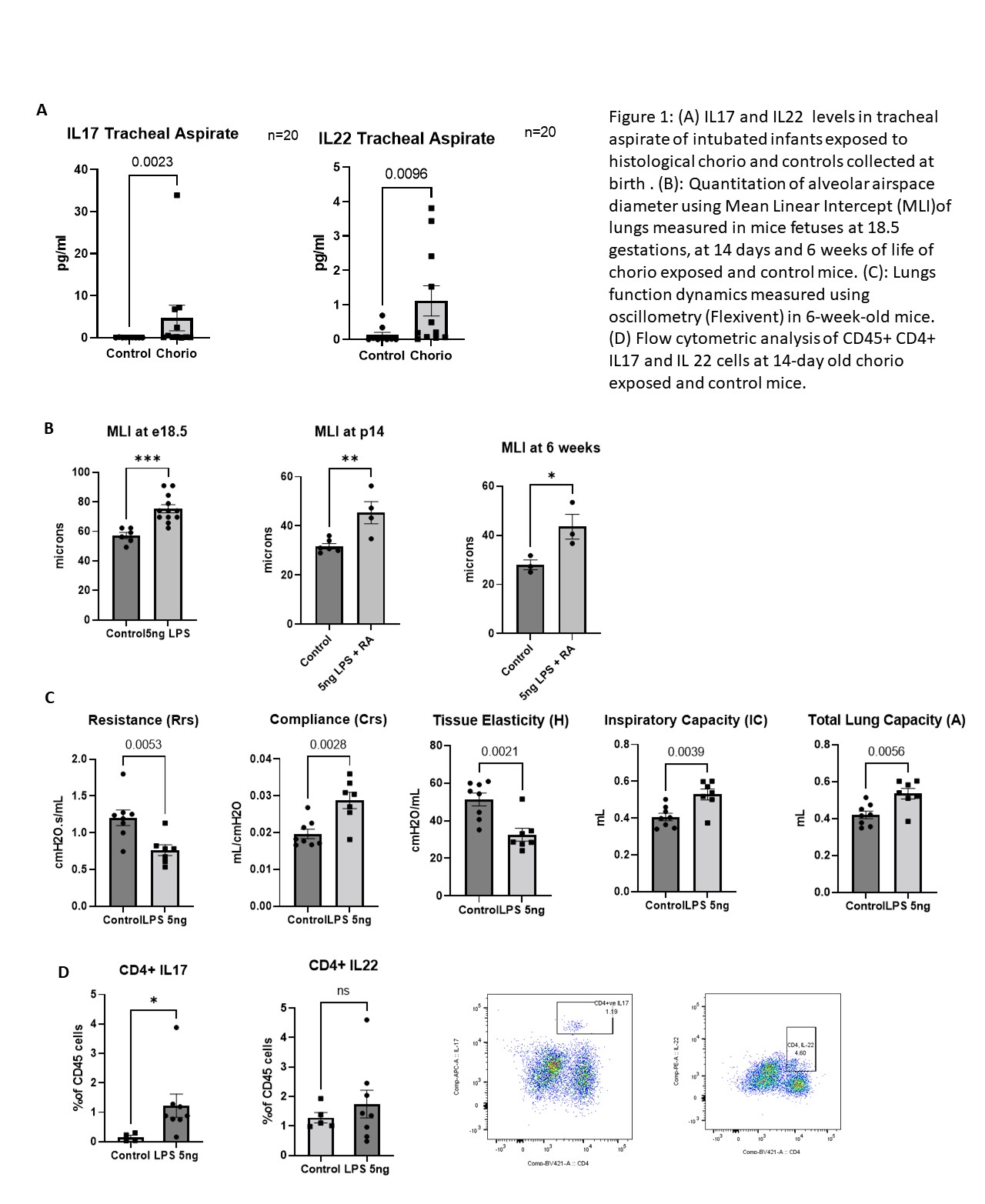
Results: There was a higher rate of BPD in preterm infants (n=43) exposed to chorio (Table 1). IL-17 and Airway pathology for chronic pulmonary diseases were the top pathways identified by Ingenuity pathway analysis (IPA) of blood transcriptome. Further, IL17a and IL22 cytokines in tracheal aspirate in a subset of preterm infants (n=20) were higher in chorio-exposed infants compared to those without chorio (Fig 1A). In the mouse model of chorio, transcriptomic analysis of chorio-exposed lungs (24 hr post LPS) also showed the IL17 pathway activation. Inhibited lung alveolarization as measured by higher mean linear intercept (MLI) was seen in chorio-exposed mice starting from E18.5 (48 hr post LPS), P14, and at 6 weeks (Fig 1B). Similarly, worse lung function, consistent with emphysema in adulthood (increased compliance, increased inspiratory capacity, decreased tissue elasticity) was observed in chorio-exposed mice at 6 weeks compared to controls (Fig 1C). Flow cytometry showed an increase in CD4+ IL17a cells in these chorio-exposed mice (Fig 1D).
Conclusion(s):
Our results indicate that IL-17 pathway activation plays an important role in propagating chorioamnionitis-induced long-term lung damage. Modulation of the IL-17 pathway may present a potential therapeutic strategy for infants exposed to chorio.