Infectious Diseases
Infectious Diseases 1
617 - Immunologic Responses and Clinical Outcomes in Pediatric Solid-Organ Transplant Recipients After SARS-CoV2 Vaccination
Publication Number: 617.225

Julia Bratic, MD, MPH (she/her/hers)
Resident
Stanford University School of Medicine
Menlo Park, California, United States
Presenting Author(s)
Background:
Pediatric solid-organ transplant recipients (SOTR) have demonstrated robust antibody- and cell-mediated immune responses to ≥3 doses of mRNA-based SARS-CoV-2 vaccine. Clinical outcomes and immune response after a fourth dose have not been well studied in this cohort.
Objective:
To quantitate antibody- and cell-mediated immune responses in pediatric SOTR and report the frequency of specific clinical outcomes including breakthrough infection and hospitalization.
Design/Methods:
Quantitative SARS-CoV-2-specific total and spike IgG, RBD (receptor binding domain)-angiotensin-converting enzyme 2 (ACE2) blocking antibody (Ab), and SARS-CoV-2-specific interferon-gamma release assay (IGRA) were measured in 5-17yo SOTR after ≥3 doses of mRNA-based SARS-CoV-2 vaccine. Positive IGRA response was defined as: Nil < =8.0 and SARS-COV-2 Antigen minus Nil (Ag response) >0.35. Severity and treatment of SARS-CoV-2 infections, allograft rejection, and immunosuppression reduction were determined through chart review and transplant team communications.
Results:
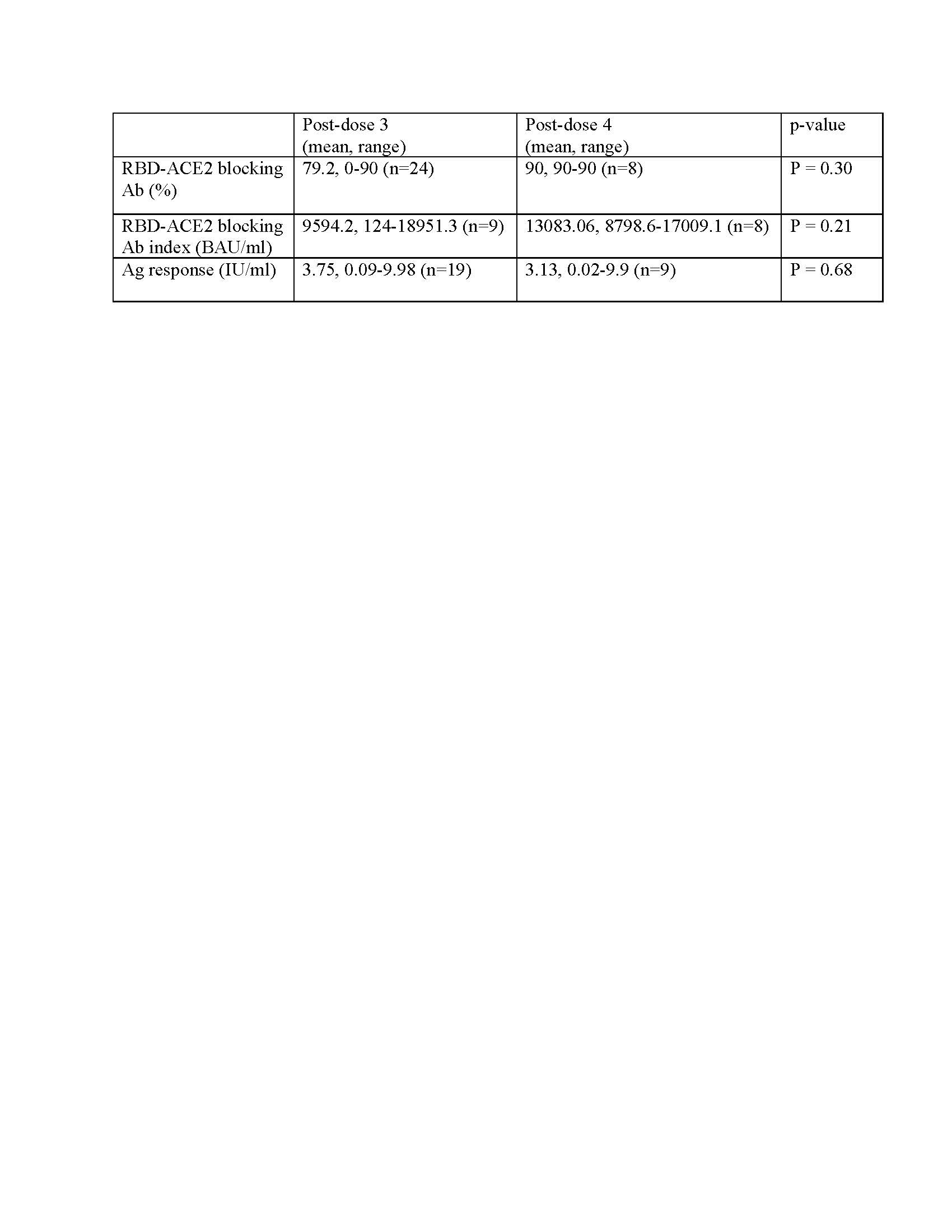
Thirty-five SOTR (liver=18, kidney=7, heart=8, lung=1, intestine=1) were enrolled. Subjects demonstrated robust RBD-ACE2 blocking Ab and Ag responses after ≥3 doses of vaccine, with no significant differences between timepoints (Table 1). No subjects had evidence of allograft dysfunction or rejection within 2 weeks after vaccination.
Of 14 subjects who had SARS-CoV-2 infection after vaccination, 2 had severe illness, both of whom were kidney transplant recipients. One hospitalized subject, treated with remdesivir, underwent immune-response testing 110 days prior to infection, showing RBD-ACE2 blocking Ab of 90% and Ag response of 9.98 IU/ml. The other had IgG testing 125 days prior to infection, showing RBD-ACE2 blocking Ab of 80%. No subjects had a reduction in immunosuppression to manage SARS-CoV-2 infection.
Mean Ag response in SARS-CoV-2-infected subjects was 3.4 IU/ml at the timepoint closest to infection, versus 2.9 IU/ml in non-infected subjects (p=0.5) in the most recent laboratory testing. RBD-ACE2 blocking Ab was not significantly different between the two groups (p=0.6, Table 2).
Conclusion(s):
Pediatric SOTR vaccinated against SARS-CoV-2 with 3 and 4 of doses nearly always mount antibody- and cell-mediated immune responses. When vaccinated, this population demonstrates favorable clinical outcomes with breakthrough disease and no need for ventilation or adjustment of immunosuppression. Future directions include durability of immune response, immune correlates of clinical protection, and larger cohorts followed longitudinally..jpg)