Neonatal Neurology: Clinical Research
Neonatal Neurology 3: Clinical 3
182 - Biomarkers of HIE: Predicting Death or Neurodevelopmental Impairment at 22-36 Months of Age
Publication Number: 182.137

Sandra E. Juul, MD, PhD (she/her/hers)
Professor of Pediatrics
University of Washington
Bellevue, Washington, United States
Presenting Author(s)
Background: The ability to predict outcomes for babies diagnosed with HIE is important for parental guidance, clinical treatment, and for stratification of patients for future neurotherapeutic studies.
Objective:
We hypothesized that one or more plasma biomarkers could predict our primary outcome of death or neurodevelopmental impairment (NDI) at 22-36 months of age better than baseline clinical data alone, and that biomarkers plus baseline clinical data would further improve prediction of the primary outcome.
Design/Methods:
This was a retrospective review of data collected from infants with moderate/severe HIE enrolled in the HEAL Trial. A sub-cohort of 180/500 infants with adequate plasma volumes at baseline (within 24 hours of birth), day 2 and day 4, and who completed a 2-year neurodevelopmental evaluation was selected for plasma biomarker evaluation. Potential biomarkers included pro-inflammatory and anti-inflammatory mediators, markers of brain injury and growth factors. NDI was defined as any of the following: cerebral palsy, GMFCS ≥1, or cognitive score < 90 on BSID-III. Poisson regression was used to assess associations between biomarkers and death or NDI. Multivariate regression models were developed and included all biomarkers selected by LASSO in at least half of the bootstrapped samples. To determine whether biomarkers improved prediction of outcomes over data available clinically at the time of study entry, we fit a model adjusted for the following clinical measurements: 5-minute Apgar, lowest pH, highest base deficit, whether there was resuscitation after 10 minutes, and sex. To evaluate how these models performed, we evaluated area under the ROC curve (AUC).
Results:
Figure 1 shows a Manhattan plot of all biomarkers evaluated, designated by category. Biomarkers from the baseline and day 4 blood samples were most likely to show a significant association with 2-year outcomes. Figure 2 shows the RR and 95% CI for biomarkers that were chosen >50% of the time by LASSO (left) and the top 5 most-chosen biomarkers (right). Figure 3 shows the comparison of ROC curves derived from each model, including a smaller curated set of biomarkers from day 1 (IL-6, c5a, NSE) and 4 (IL-8, UCHL1, Tau). Biomarkers from baseline (AUC 0.887) and day 4 (AUC 0.900) both significantly increased model performance over baseline clinical data (BCD) alone (AUC 0.838), while the curated biomarkers had the highest AUC 0.904.
Conclusion(s):
Biomarkers measured at baseline, day 4, or the curated set improved prediction of 2-year neurodevelopmental outcomes in this high-risk population over baseline clinical data alone.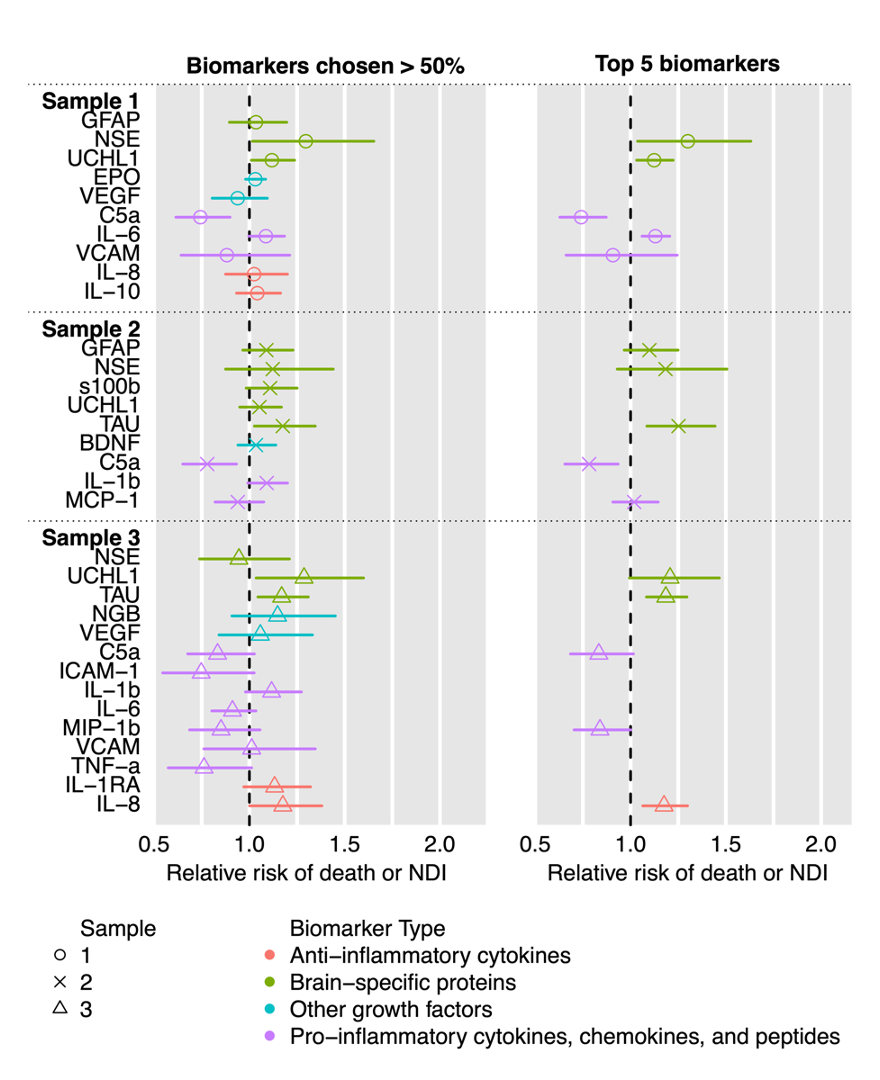
.png)
.png)
